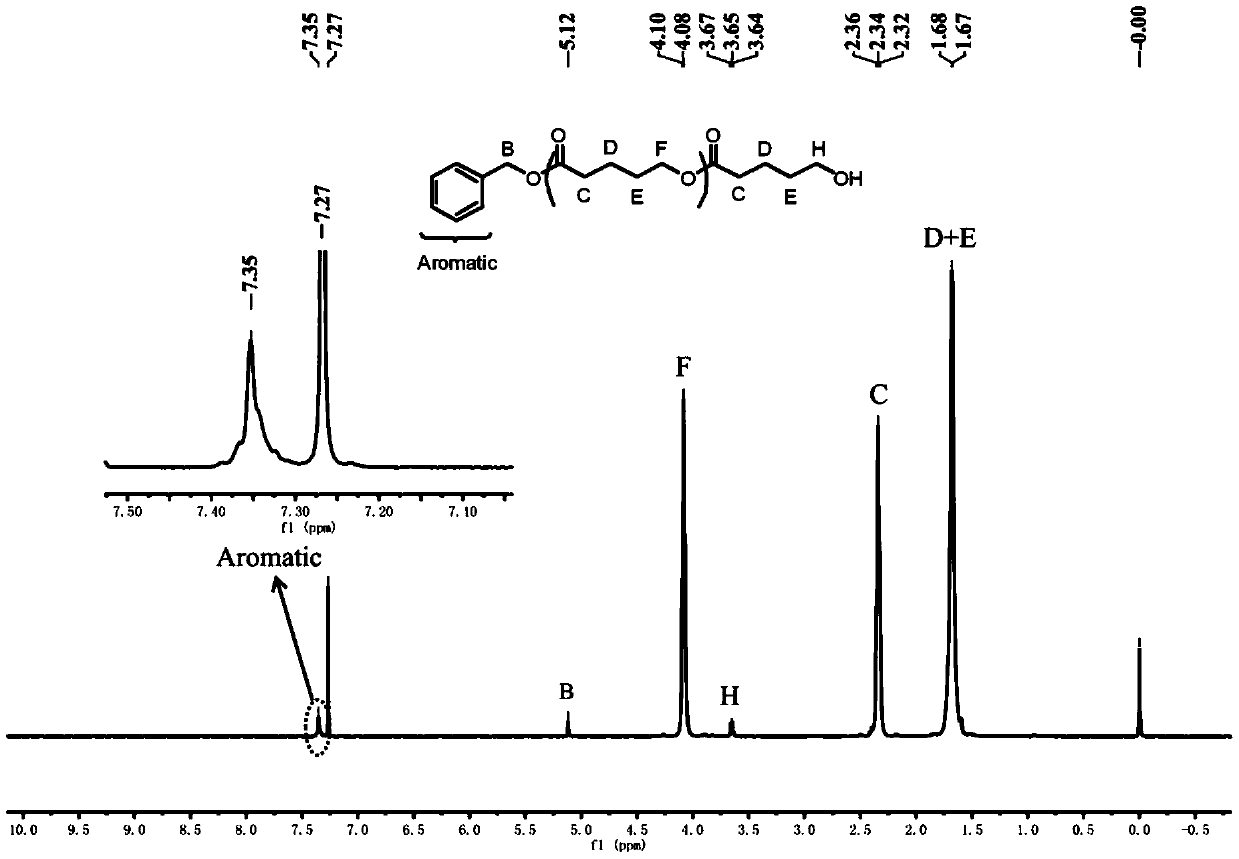
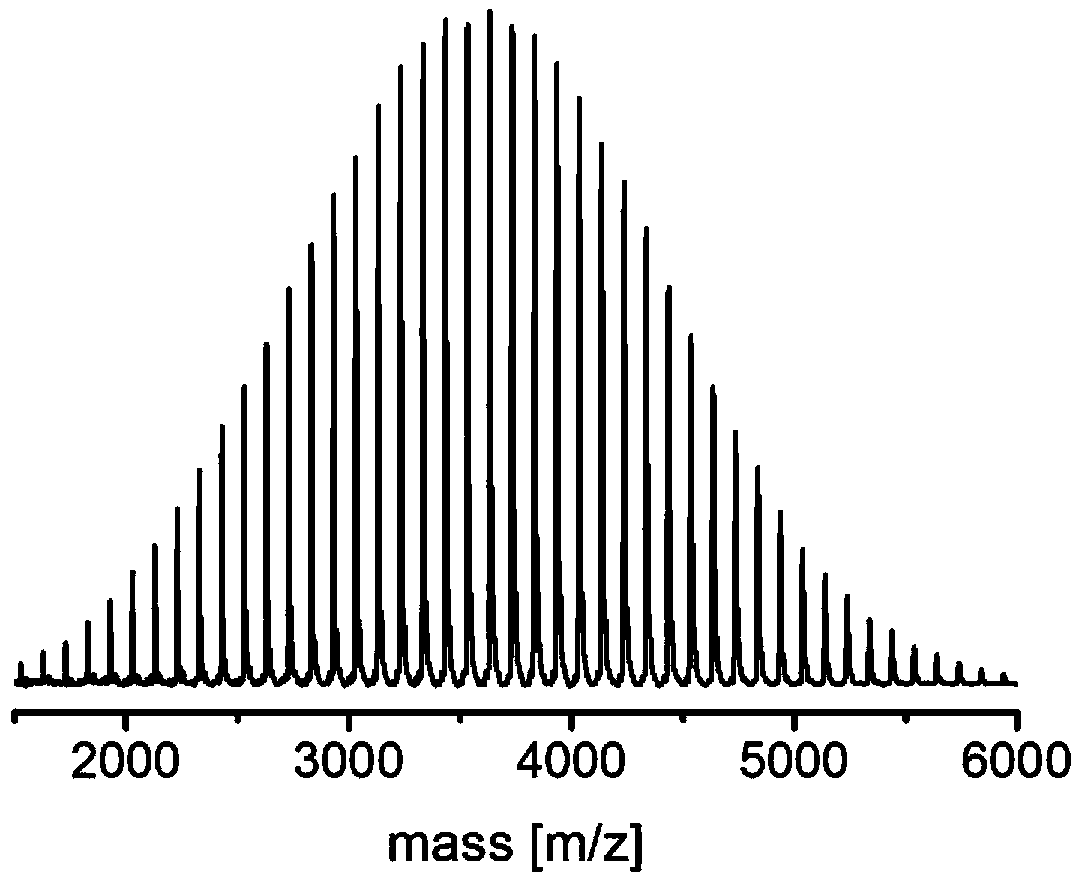
Metal free catalyst system for synergistic catalysis of lactone ring opening polymerization in organic solvent
A technology of metal-free catalysis and synergistic catalysis, applied in the field of polymer synthetic materials, can solve the problems of poor catalytic activity and low conversion rate, and achieve the effects of fast reaction, high conversion rate, and simple and easy-to-operate polymerization process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
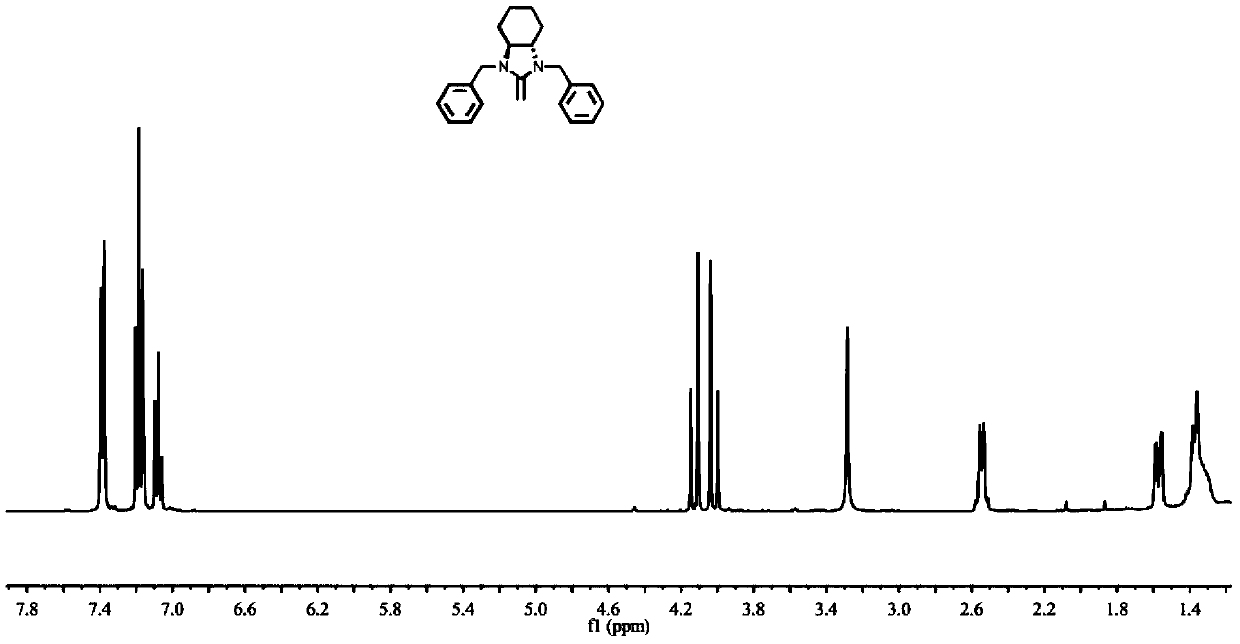
[0046] Embodiment 1: Synthesis of N-heterocyclic exoolefin NHO 1
[0047] The reaction formula of synthetic N-heterocyclic exoolefin NHO 1 is as follows:
[0048]
[0049] Under argon or nitrogen atmosphere, 50mL Schlenk reaction tube is pumped and baked three times, dry compound Pre 1 (2.38g, 10mmol), 5mL newly steamed anhydrous tetrahydrofuran is added in the 50mL Schlenk reaction tube, the tetrahydrofuran solution (THF) of injection KHMDS ( 10 mL, 1M in THF), stirred at room temperature for 6 h, filtered, and vacuum-dried to constant weight to obtain yellow solid NHO 1 (0.73 g, yield=66%). 1 H NMR (400MHz, d 8 -Toluene)δ(ppm)5.32(s,2H,N-CH=CH-N),2.55(s,2H,-C=CH 2 ),2.41(s,6H,N-CH 3 ). 13 C NMR (400MHz, d 8 -Toluene) δ (ppm) 112.54, 39.92, 32.07.
Embodiment 2
[0050] Embodiment 2: Synthesis of N-heterocyclic exoolefin NHO 2
[0051] Synthetic N-heterocyclic exoolefin NHO The reaction formula is as follows:
[0052]
[0053] Under argon or nitrogen atmosphere, the 10mL Schlenk reaction tube was pumped and baked three times, the dry compound Pre 2 (0.53g, 2mmol), 3mL freshly steamed anhydrous tetrahydrofuran was added in the 10mL Schlenk reaction tube, and the THF solution of KHMDS ( 2mL, 1M in THF), stirred at room temperature for 6h, filtered, and vacuum-dried to constant weight to obtain yellow solid NHO 2 (0.26g, yield=19%). 1 H NMR (400MHz, d 8 -Toluene)δ(ppm)2.67(s,2H,-C=CH 2 ),2.53(s,6H,N-CH 3 ),1.42(s,6H,C-CH 3 ). 13 C NMR (400MHz, d 8 -Toluene) δ (ppm) 153.11, 113.83, 40.12, 28.81, 8.16.
Embodiment 3
[0054] Embodiment 3: Synthesis of N-heterocyclic exoolefin NHO 3
[0055] The reaction formula of synthetic N-heterocyclic exoolefin NHO is as follows:
[0056]
[0057] Under argon or nitrogen atmosphere, the 25mL Schlenk reaction tube was pumped and baked three times, the dry compound Pre 3 (1.47g, 5mmol), 5mL of newly steamed anhydrous THF were added in the 25mL Schlenk reaction tube, and the THF solution of KHMDS ( 5mL, 1M in THF), stirred at room temperature for 6h, filtered, and vacuum-dried to constant weight to obtain yellow solid NHO 3 (0.64g, yield=77%). 1 H NMR (400MHz, d 8 -Toluene)δ(ppm)2.59(s,6H,N-CH 3 ),1.86(s,6H,C-CH 3 ), 1.47(s,6H,-C=CCH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com