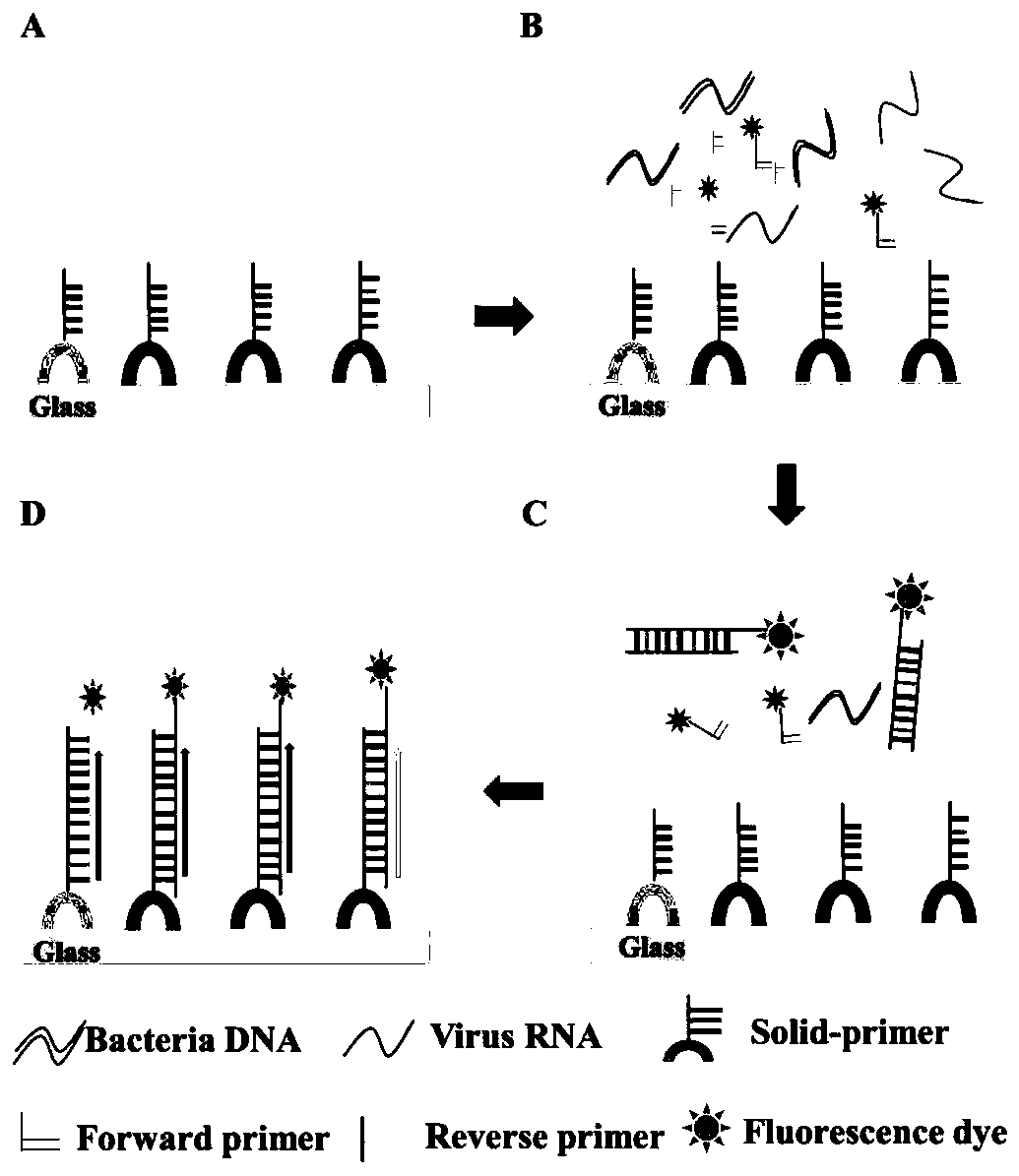
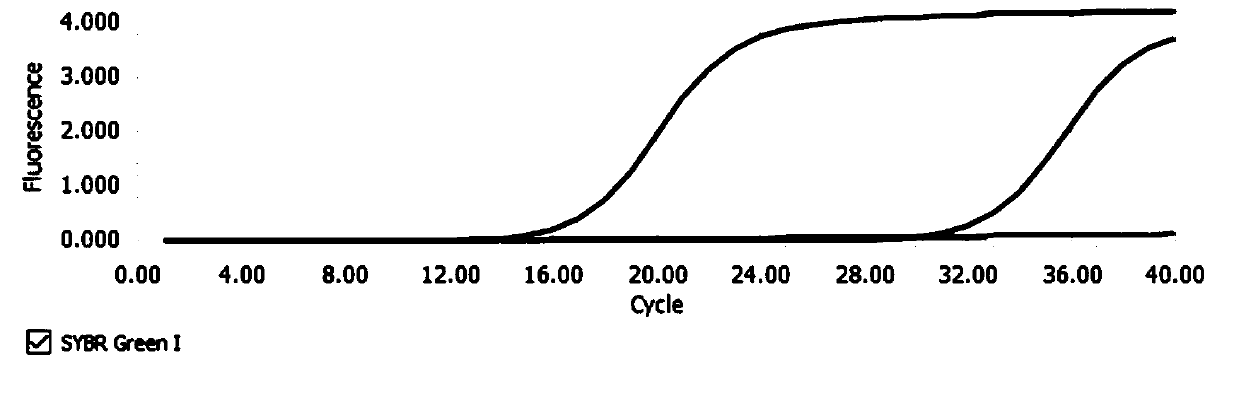
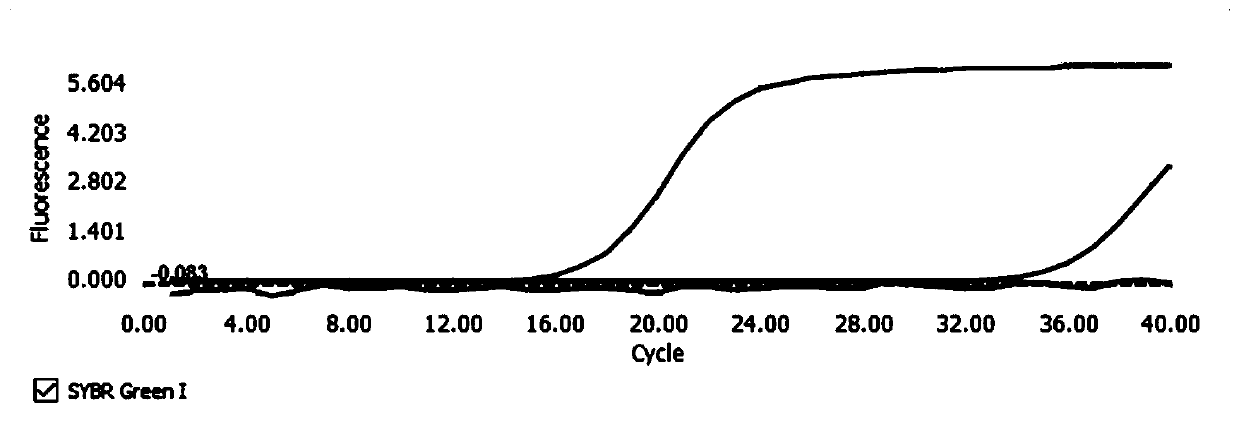
Reagent kit capable of simultaneously detecting virulent viruses and bacteria and detecting method
A kit, virus technology, applied in the field of virus detection and molecular biology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Through the analysis of the conservation and specificity of the genome sequences of Ebola virus, Xinjiang hemorrhagic fever virus, Brucella and Bacillus anthracis, and through the screening of a large number of detection target genes and specific primers and probes, a high Primers and probes for specificity and amplification efficiency.
[0094] The present embodiment provides the combination of primers and probes for simultaneous detection of Ebola virus, Xinjiang hemorrhagic fever virus, Brucella and Bacillus anthracis, specifically as follows:
[0095] The specific primer sequence for detecting Ebola virus is shown in SEQ ID NO.5 and SEQ ID NO.6, and the probe sequence is shown in SEQ ID NO.13:
[0096] SEQ ID NO.5: GTGCGAATAACTATGAGGAAG;
[0097] SEQ ID NO. 6: TGATGCCCTTGCCCCCT;
[0098] SEQ ID NO. 13: GGTTTGTTTCAGAGCCATATCACCAAGA.
[0099] The specific primer sequences used to detect Xinjiang hemorrhagic fever virus are shown in SEQ ID NO.7 and SEQ ID NO.8, and ...
Embodiment 2
[0113] The present embodiment provides a kit for simultaneously detecting Ebola virus, Xinjiang hemorrhagic fever virus, Brucella and Bacillus anthracis, and the specific composition is as shown in Table 1:
[0114] Table 1 Composition of the kit (24T)
[0115]
[0116] Wherein, the composition of PCR reaction liquid is as follows: 50mM Tris-HCl, 75mM KCl, 3mM MgCl 2 , 10mM DTT, 2.5mM dUTP;
[0117] The concentration of the specific primer mixture for Ebola virus, Xinjiang hemorrhagic fever virus, Brucella and Bacillus anthracis is 50 μM;
[0118] The composition of the enzyme system is as follows: 0.1U / μl Hitaq hot-start DNA polymerase, 0.2U / μl UNG enzyme, 2.4U / μl super M-MLV reverse transcriptase, 0.32U / μl IRNasin RNase inhibitor.
[0119] In the above kit, the preparation method of virus-like particles containing Ebola virus NP gene and Xinjiang hemorrhagic fever virus S gene as a positive quality control is as follows:
[0120] (1) Download the genome sequences of Eb...
Embodiment 3
[0146] The present embodiment provides a kind of method that utilizes the kit that embodiment 2 provides to carry out Ebola virus, Xinjiang hemorrhagic fever virus, Brucella and Bacillus anthracis synchronous detection, comprising the steps:
[0147] (1) Sample collection and processing
[0148] Blood samples were collected in EDTA blood collection tubes and mixed thoroughly. Samples can be used for detection immediately; if not detected immediately, samples should be stored at 4°C for no more than 24 hours, -20°C for no more than 3 months, -70°C for long-term storage, and repeated freezing and thawing no more than 5 times.
[0149] (2) Extraction of sample nucleic acid
[0150] Use commercially available nucleic acid extraction kits, such as QIAampMinElute Virus Spin Kit from QIAGEN (Cat. No.: 57704), Viral Genomic DNA / RNA Extraction Kit from Tiangen Biochemical Technology Co., Ltd. (Cat. No.: DP315) or TaKaRa MiniBEST Viral RNA / DNA Extraction Kit Ver.5.0 (Product No.: 9766...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com