Method for selectively preparing different cyclic fullerene derivatives in iodine-alkali system
A fullerene derivative and fullerene technology are applied in the field of preparation of fullerene derivatives, which can solve the problems of decreased yield and increased toluene insoluble matter, and achieve high conversion rate, less toluene insoluble matter, and higher yield. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
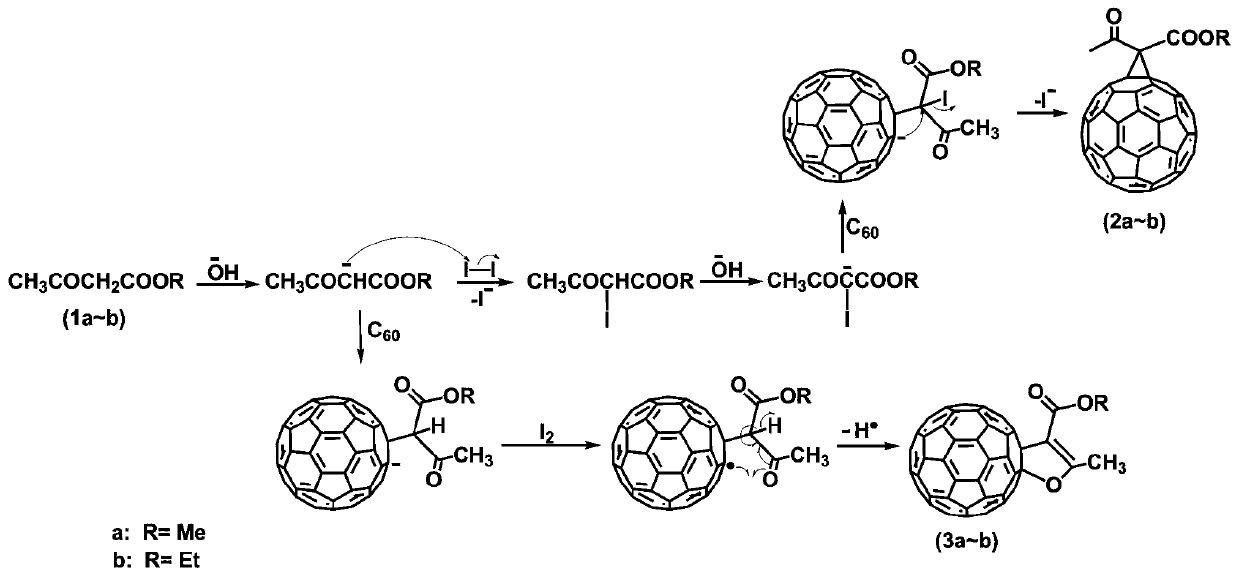
[0050] The method for selectively preparing methylene bridged fullerene derivatives in an iodine-alkali system under mild conditions proposed by the present invention comprises the following steps:
[0051] S1: 36mg C 60 (50μmol), 50 times the chemical equivalent of methyl acetoacetate (2.5mmol) and 5 times the chemical equivalent of iodine were added to 15mL o-dichlorobenzene (o-DCB) solution (or toluene solution), and an inert gas (argon) or nitrogen), stirred at room temperature for 15 min, and then added 3 times the chemical equivalent of tetrabutylammonium hydroxide (TBAOH) (1.0M methanol solution) under the protection of an inert gas, and reacted for 0.5 h to end the reaction. The solvent was removed by rotary evaporation, and the crude product was obtained after washing and filtering with methanol.
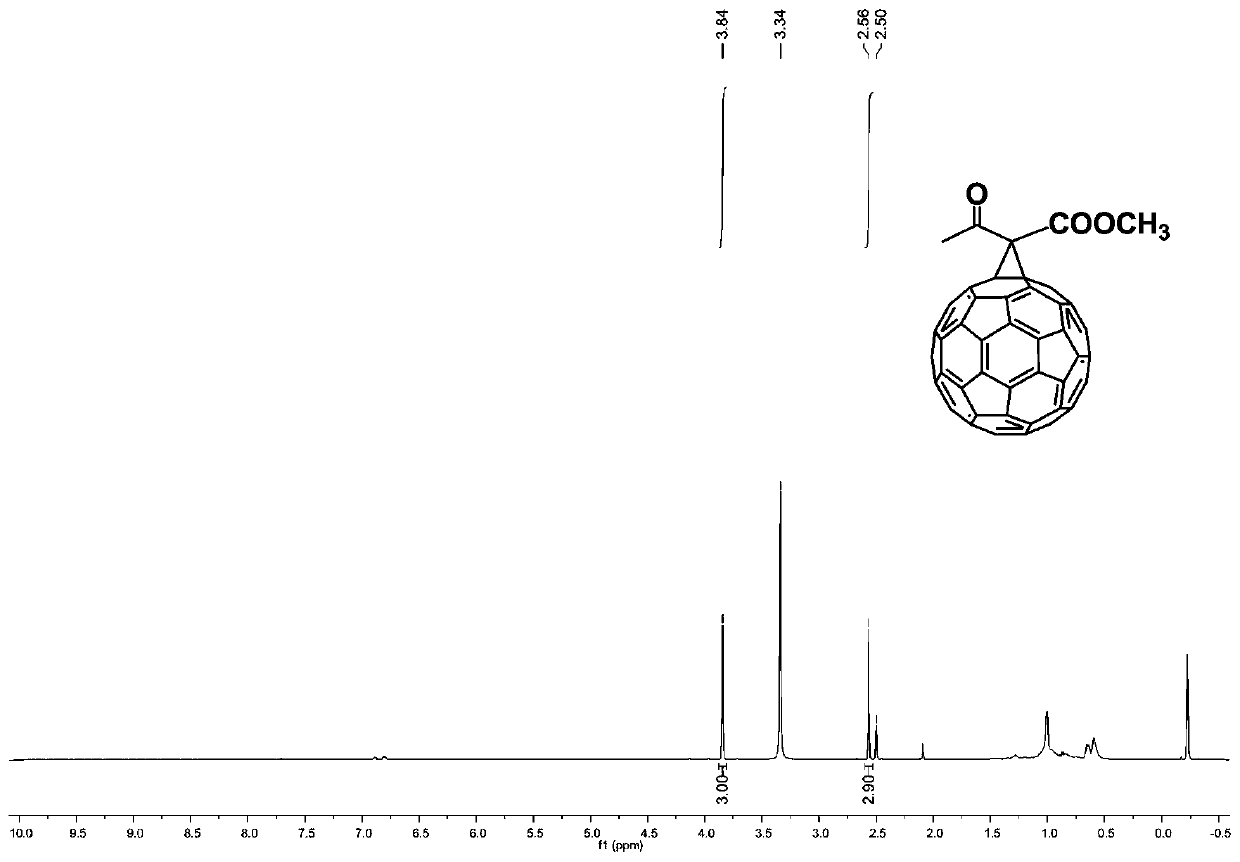
[0052] S2: The crude product can be separated and purified by silica gel column chromatography to obtain product 2a, and the eluent system is n-hexane: carbon disulfide: t...
Embodiment 2
[0056] The method for selectively preparing methylene bridged fullerene derivatives in an iodine-alkali system under mild conditions proposed by the present invention comprises the following steps:
[0057] S1: 36mg C 60 (50μmol), 50 times the chemical equivalent of ethyl acetoacetate (2.5mmol) and 5 times the chemical equivalent of iodine were added to 15mL o-dichlorobenzene (o-DCB) solution (or toluene solution), and an inert gas (argon) or nitrogen), stirred at room temperature for 15 min, and then added 3 times the chemical equivalent of tetrabutylammonium hydroxide (TBAOH) (1.0M methanol solution) under the protection of an inert gas, and reacted for 0.5 h to end the reaction. The solvent was removed by rotary evaporation, and the crude product was obtained after washing and filtering with methanol.
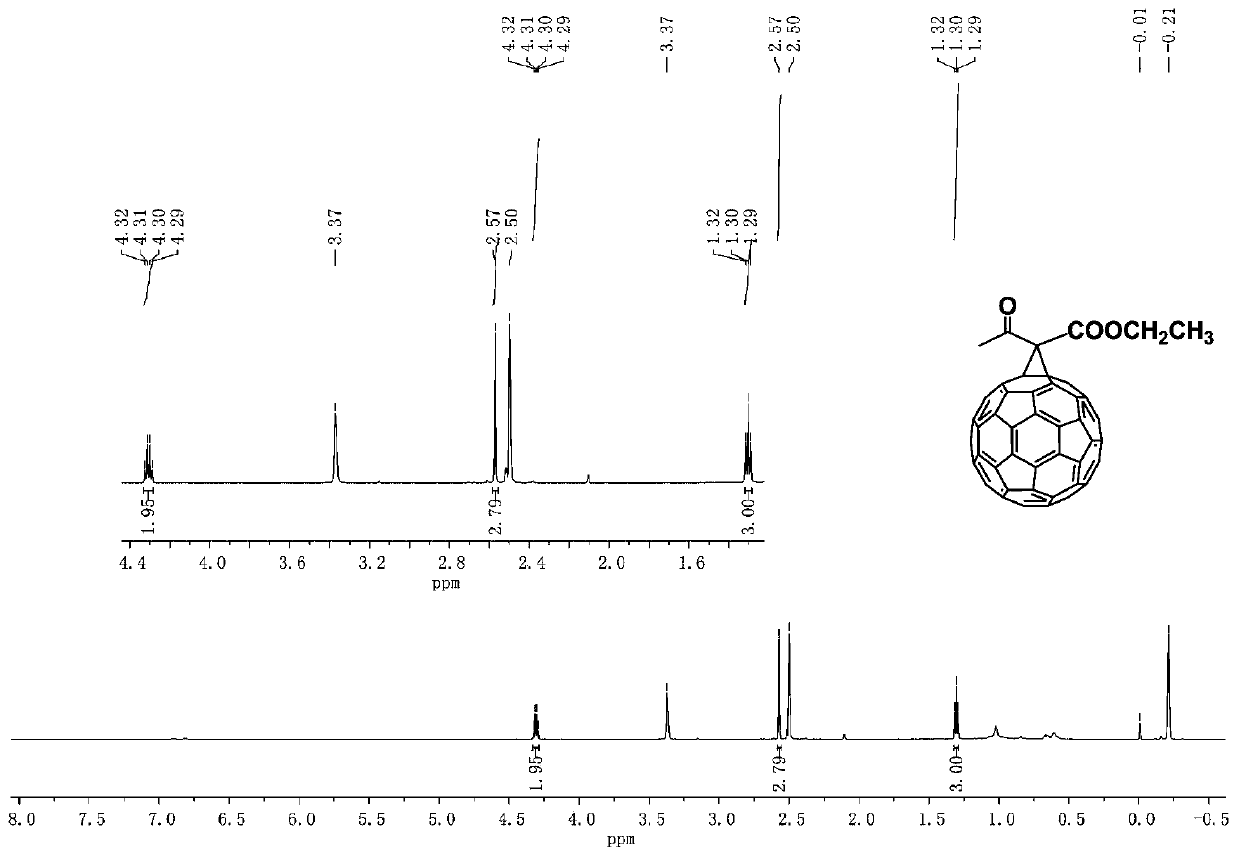
[0058] S2: The crude product can be separated and purified by silica gel column chromatography to obtain product 2b. The eluent system is n-hexane: carbon disulfide: toluen...
Embodiment 3
[0067] The method for selectively preparing dihydrofuran ring-fused fullerene derivatives in an iodine-alkali system under mild conditions proposed by the present invention comprises the following steps:
[0068] S1: 36mg C 60 (50 μmol), 50 times the chemical equivalent of methyl acetoacetate (2.5mmol) was added to 15mL of o-dichlorobenzene (o-DCB) solution (or toluene solution), and an inert gas (argon or nitrogen) was passed through. Stir at high temperature for 15 minutes, then add 1 times the chemical equivalent of tetrabutylammonium hydroxide (TBAOH) (1.0M methanol solution) under the protection of an inert gas. After reacting for 0.5 hours, add 3 times the chemical equivalent of iodine and continue the reaction for 0.5 hours Then end the reaction. The solvent was removed by rotary evaporation, and the crude product was obtained after washing and filtering with methanol.
[0069] S2: The crude product can be separated and purified by silica gel column chromatography to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com