A preparation method of zinc lithium titanate/carbon nanocomposite negative electrode material for lithium ion battery
A lithium-ion battery, carbon nanocomposite technology, applied in battery electrodes, secondary batteries, circuits, etc., can solve the problems of easy formation of SEI film, poor cycle stability, weak electrical conductivity, etc., to achieve good electrochemical performance and improve electrical conductivity. Performance, the effect of reducing side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
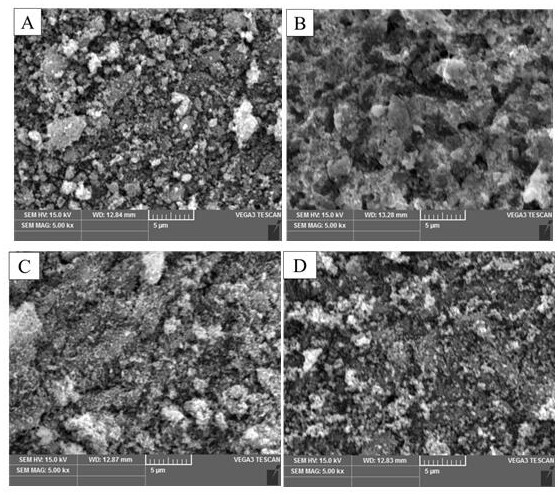
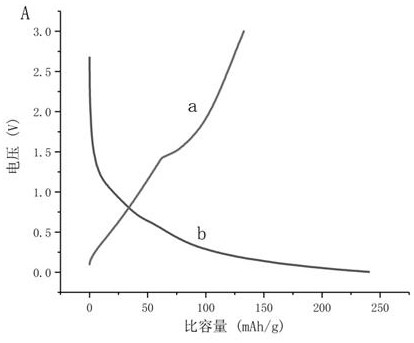
Examples
Embodiment 1
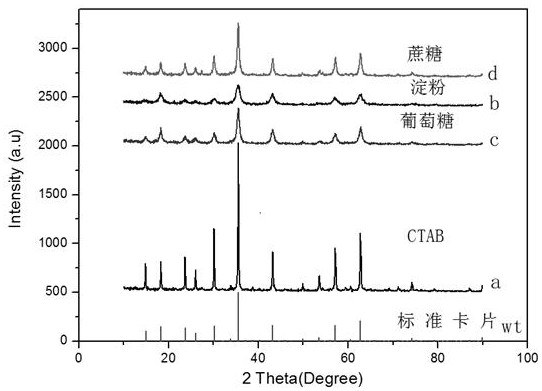
[0041] 1) Weigh 0.03mol CTAB and dissolve it in a beaker containing 200mL deionized water, then weigh 0.005mol Li 2 CO 3 and 0.005mol Zn(CH 3 COO) 2 • 2H 2 O was added to the beaker and magnetically stirred for 30 min to obtain a mixed solution. Weigh 0.015mol C 16 h 36 o 4 Ti was diluted with an equal volume of absolute ethanol, slowly added to the above mixed solution, and ultrasonically treated for 20 minutes;
[0042] 2) Put the beaker into the oil bath and heat up to 60°C and stir for 2 hours, then heat up to 80°C and stir for 4 hours until the water in the sol evaporates and gel appears. Take out the beaker and let it stand for 12 hours, then put the beaker containing the wet gel in the Dry in an oven at 110°C 2 ZnTi 3 o 8 Precursor of / C;
[0043] 3) will Li 2 ZnTi 3 o 8 / C precursor is ground to nanometer size, and then Li 2 ZnTi 3 o 8 / C powder is evenly placed in a quartz boat and placed in a microwave tube furnace. In an argon atmosphere, it is trea...
Embodiment 2
[0045] 1) Weigh 0.03mol soluble starch and dissolve it in a beaker containing 200mL deionized water, then weigh 0.005mol Li 2 CO 3 and 0.005mol Zn(CH 3 COO) 2 • 2H 2 O was added to the beaker, and magnetically stirred for 30 min to obtain a mixed solution. Weigh 0.015mol C 16 h 36 o 4 Ti was diluted with an equal volume of absolute ethanol, slowly added to the above mixed solution, and ultrasonically treated for 20 minutes;
[0046] 2) Put the beaker into the oil bath and heat up to 60°C and stir for 2 hours, then heat up to 80°C and stir for 4 hours until the water in the sol evaporates and gel appears. Take out the beaker and let it stand for 12 hours, then put the beaker containing the wet gel in the Dry in an oven at 110°C 2 ZnTi 3 o 8 Precursor of / C;
[0047] 3) will Li 2 ZnTi 3 o 8 / C precursor is ground to nanometer size, and then Li 2 ZnTi 3 o 8 / C powder is evenly placed in a quartz boat and placed in a microwave tube furnace. In an argon atmosphere,...
Embodiment 3
[0049] 1) Weigh 0.03mol glucose and dissolve it in a beaker containing 200mL deionized water, then weigh 0.005mol Li 2 CO 3 and 0.005mol Zn(CH 3 COO) 2 • 2H 2 O was added to the beaker, and magnetically stirred for 30 min to obtain a mixed solution. Weigh 0.015mol C 16 h 36 o 4 Ti was diluted with an equal volume of absolute ethanol, slowly added to the above mixed solution, and ultrasonically treated for 20 minutes;
[0050] 2) Put the beaker into the oil bath and heat up to 60°C and stir for 2 hours, then heat up to 80°C and stir for 4 hours until the water in the sol evaporates and gel appears. Take out the beaker and let it stand for 12 hours, then put the beaker containing the wet gel in the Dry in an oven at 110°C 2 ZnTi 3 o 8 Precursor of / C;
[0051] 3) will Li 2 ZnTi 3 o 8 / C precursor is ground to nanometer size, and then Li 2 ZnTi 3 o 8 / C powder is evenly placed in a quartz boat and placed in a microwave tube furnace. In an argon atmosphere, it is ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| discharge efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com