Method for preparing 2,5-acetonyl acetone
A technology of hexanedione and acidic aqueous solution is applied in the preparation of heterocyclic compounds, the preparation of carbonyl compounds by hydrolysis, organic chemistry, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-21
[0024] Grind and dry willow, pine, mulberry, birch, newspaper, cotton, cotton thread, cotton cloth, etc., take 20g and add it to a 4L reaction kettle, add 2g of 5% Pd / C, add 0.4L of 36% hydrochloric acid aqueous solution and 1.2 L of chloroform, filled with 6MPa hydrogen, reacted at 100°C for 2 hours.
[0025] Table 1 Different raw materials for preparing 2,5-hexanedione
[0026]
[0027] Note: It can be seen from Example 12 that lignin cannot generate 2,5-hexanedione, and hemicellulose rich in five-carbon sugars will generate a large amount of furfural, and its further conversion products are also five-carbon Levulinic acid, thus theoretically impossible to convert the five-carbon sugar directly into 2,5-hexanedione containing six carbon atoms. Therefore, the yield of 2.5-hexanedione in the above table is calculated based on the mass of the six-carbon sugar in each raw material, and the following tables all calculate the yield of 2,5-hexanedione according to this method. ...
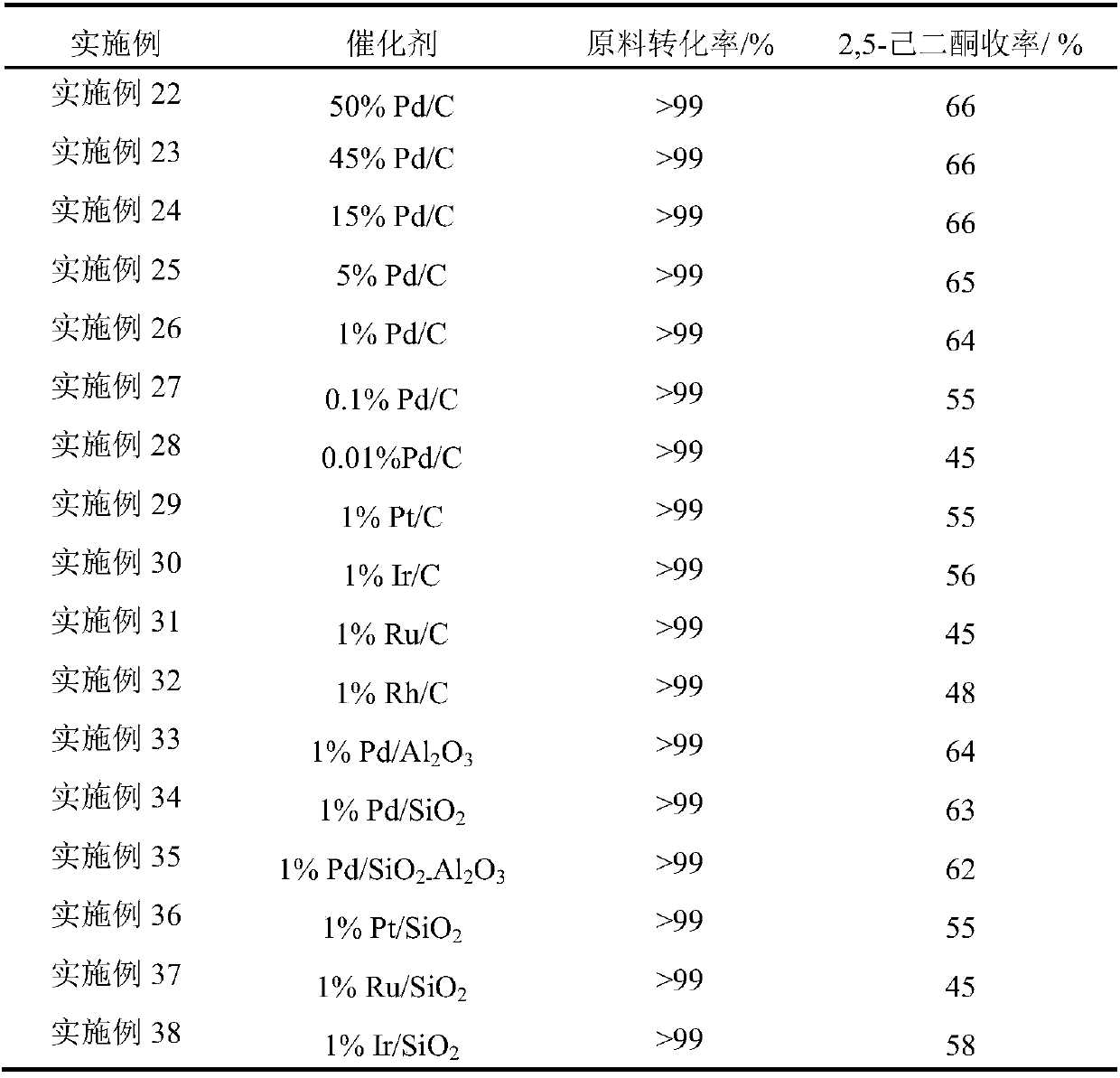
Embodiment 22-38
[0031] Put 20g of cellulose into a 4L reactor, add 2g of noble metal catalyst, add 0.4L of 36% hydrochloric acid aqueous solution and 1.2L of chloroform, fill with 6MPa hydrogen, and react at 100°C for 2 hours.
[0032] Table 2 Different catalysts prepare 2,5-hexanedione
[0033]
[0034] It can be seen from Table 2 that catalysts with different contents, different supports and different noble metal supports can effectively catalyze the reaction, among which Pd has the best activity among all noble metals; among all supports, activated carbon and alumina are the most active. Good; when the metal loading is greater than 1%, an ideal yield of 2,5-hexanedione can be obtained.
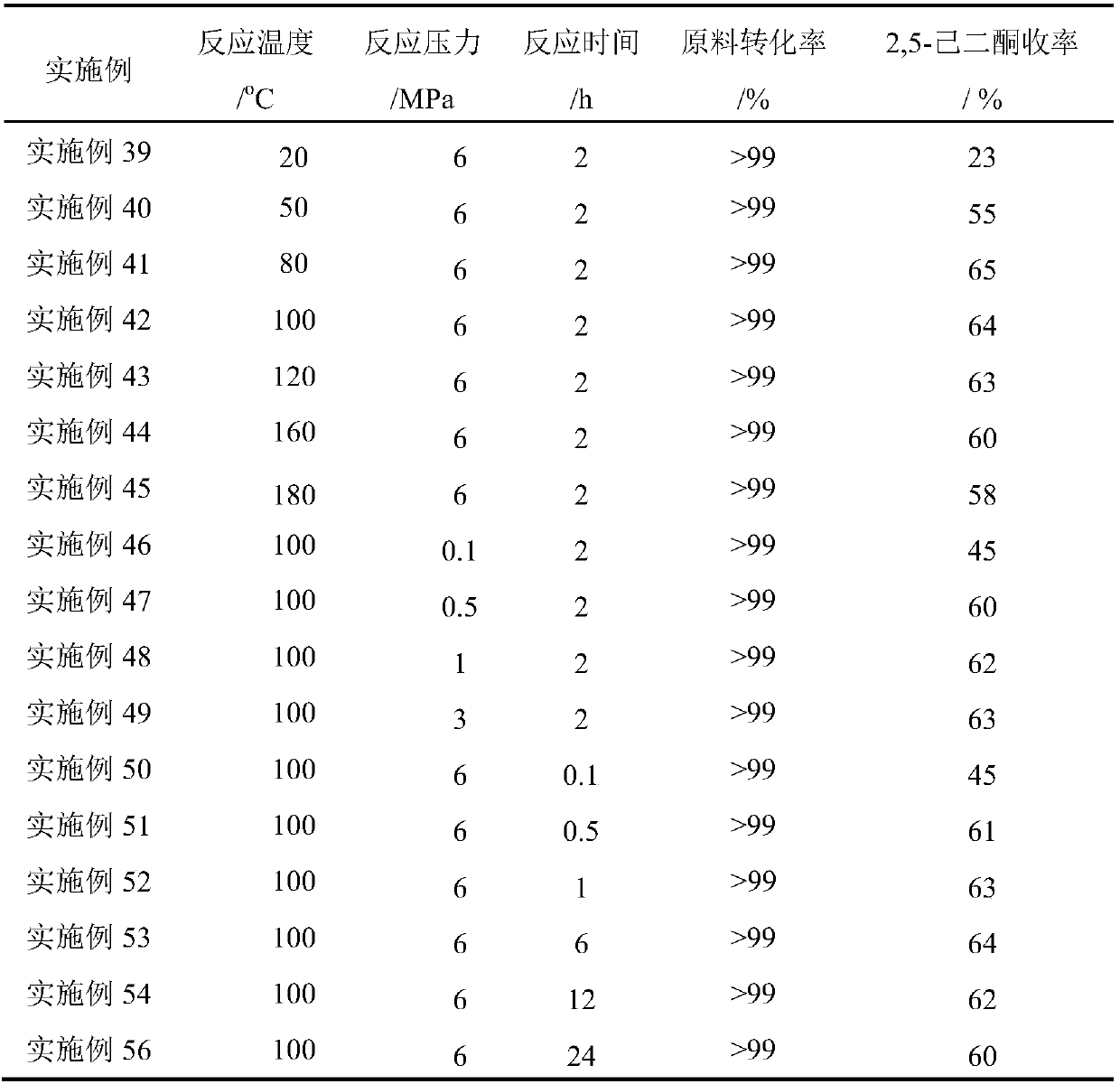
Embodiment 39-56
[0036] Put 20g of cellulose into a 4L reactor, add 2g of 1% Pd / C catalyst, add 0.4L of 36% aqueous hydrochloric acid and 1.2L of chloroform, fill in a certain pressure of hydrogen, and react at different reaction temperatures certain hours.
[0037] Table 3 Different reaction conditions prepare 2,5-hexanedione
[0038]
[0039] As can be seen from Table 3, the reaction can be effectively catalyzed under different reaction times, different hydrogen pressures and different reaction temperatures. Even at room temperature, a 23% yield of 2,5-hexanedione can be obtained, increasing the reaction temperature can increase the yield of 2,5-hexanedione, and the maximum yield is 65% at 80 degrees. Continue to increase the reaction temperature, the yield of 2,5-hexanedione decreases slightly, indicating that under high temperature conditions, 2,5-hexanedione will be further converted into other substances. When the reaction time is 0.1 hour, there is already a 45% yield of 2,5-hexane...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com