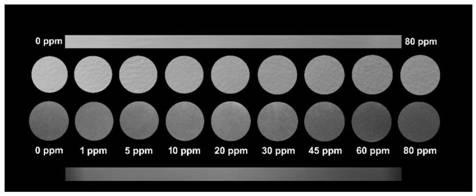
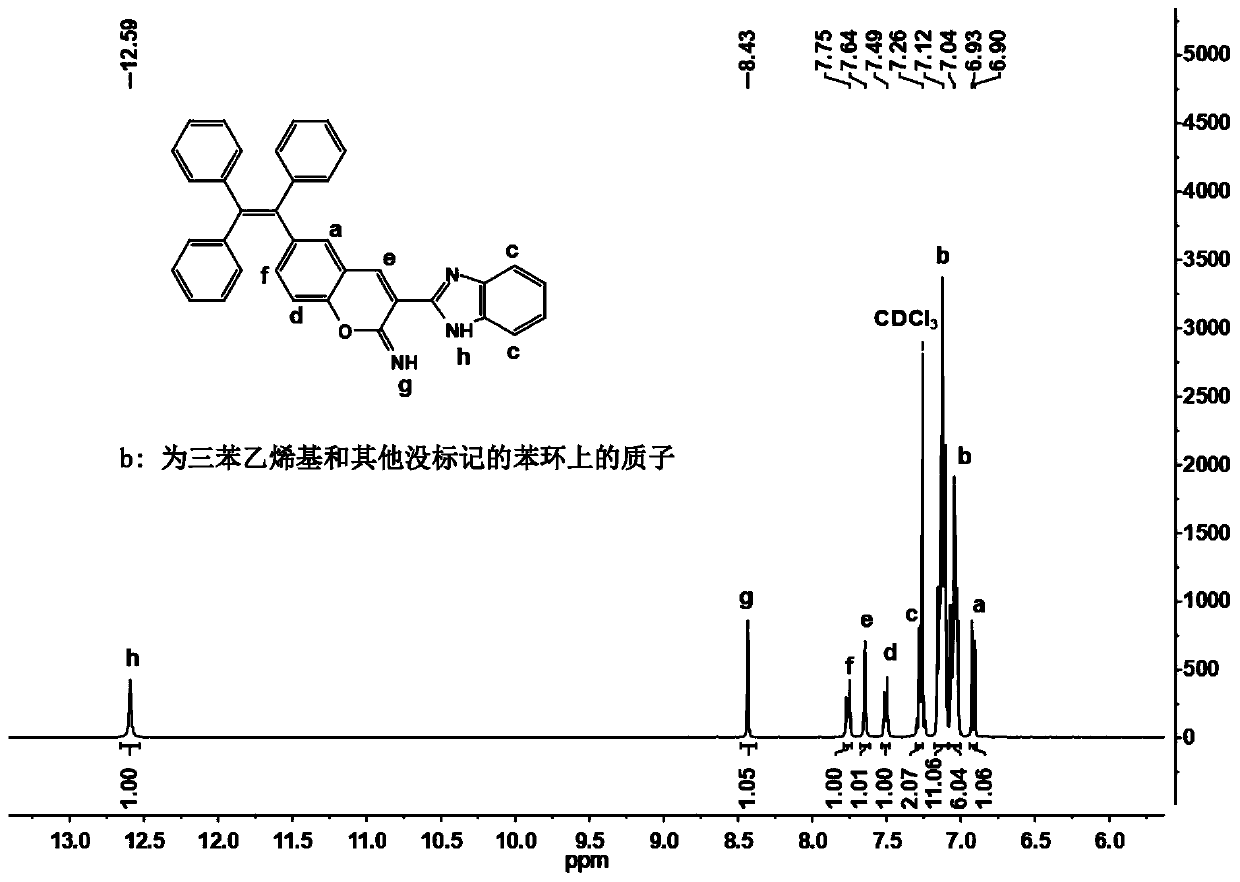
Ratio fluorescent probe as well as preparation method and application thereof
A fluorescent probe, ratio-based technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of limited probe practical application, insignificant color change, long probe response time, etc. The effect of promoting application, high sensitivity and accuracy, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] The present invention also provides a method for preparing a ratiometric fluorescent probe, comprising the following steps:
[0044] a) Mix triphenylbromoethylene and 3-formyl-4-hydroxyphenylboronic acid pinacol ester in the presence of toluene, potassium carbonate solution and tetrabutylammonium bromide, and then add a catalyst for the first reaction , compound A is obtained after the first separation and purification; the catalyst is tetrakis (triphenylphosphine) palladium (0);
[0045] The compound A has a structure shown in formula (II):
[0046]
[0047] b) The compound A obtained in step a) and 2-benzimidazole acetonitrile are reacted for the second time in the presence of ethanol and piperidine, and the ratiometric fluorescent probe is obtained after the second separation and purification;
[0048] The ratiometric fluorescent probe has a structure shown in formula (I):
[0049]
[0050] The present invention first mixes triphenylbromoethylene and 3-formyl...
Embodiment 1
[0079] (1) Dissolve 670mg of triphenylbromoethylene in 20mL of toluene, add 4mL of potassium carbonate solution and 50mg of tetrabutylammonium bromide under stirring, then add 500mg of 3-formyl-4-hydroxyphenylboronic acid pinacol ester, stirred at room temperature for 20 min, and finally added 10 mg of catalytic amount of tetrakis(triphenylphosphine) palladium (0), heated to 90°C for 24 h under nitrogen protection, added ethyl acetate after cooling, washed with water 3 times, and organically The phase was dried over anhydrous sodium sulfate and concentrated, and the reaction crude product was purified by column chromatography to obtain 306 mg of light yellow compound A (yield 41%).
[0080] (2) Dissolve 188mg of compound A and 78.5mg of 2-benzimidazole acetonitrile in 7.5mL of ethanol, add 40μL of piperidine, heat to 80°C under nitrogen protection for 1h, filter after cooling to room temperature, and wash with ethanol for 3 times After vacuum drying, 190.5 mg of milky white pr...
Embodiment 2
[0083] (1) Dissolve 670 mg of triphenylbromoethylene in 25 mL of toluene, add 6 mL of potassium carbonate solution and 70 mg of tetrabutylammonium bromide under stirring, and then add 600 mg of 3-formyl-4-hydroxyphenylboronic acid pinacol ester, stirred at room temperature for 30 min, and finally added 15 mg of catalytic amount of tetrakis(triphenylphosphine) palladium (0), heated to 95 °C for 22 h under nitrogen protection, added ethyl acetate after cooling, washed with water for 3 times, and organically The phase was dried and concentrated over anhydrous sodium sulfate, and the crude reaction product was purified by column chromatography to obtain 331 mg of pale yellow compound A (yield 44%).
[0084] (2) Dissolve 188mg of Compound A and 86.5mg of 2-benzimidazole acetonitrile in 9mL of ethanol, add 50μL of piperidine, heat to 82°C under nitrogen protection and reflux for 1.2h, cool to room temperature, filter, and wash with ethanol for 3 times After vacuum drying, 198.5 mg o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com