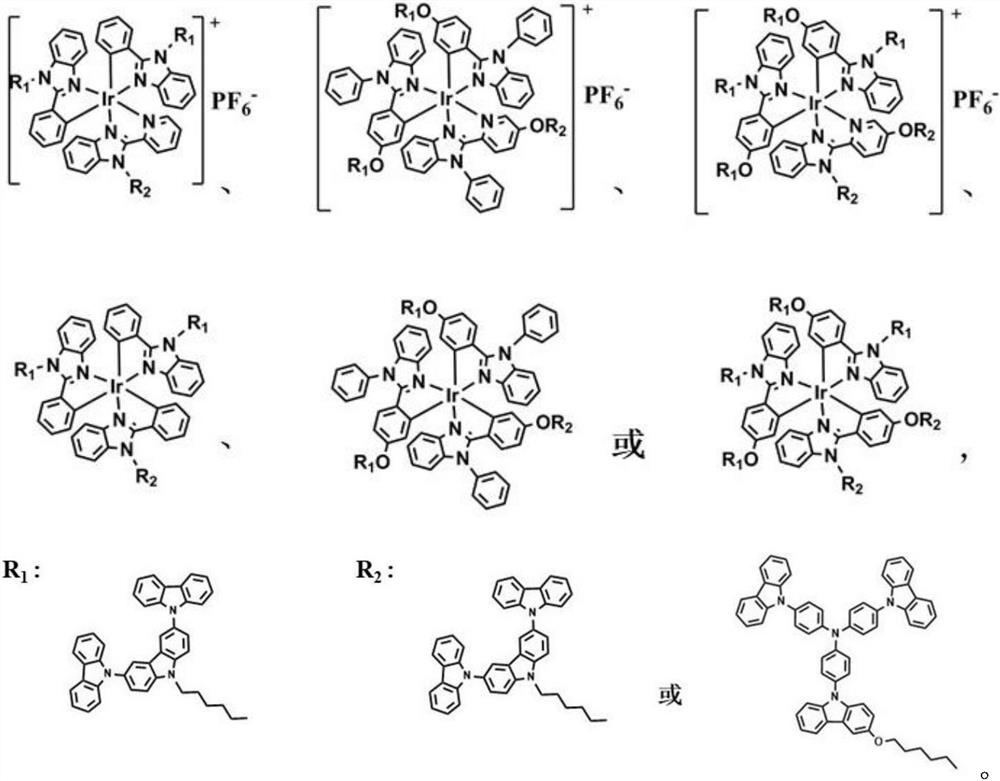
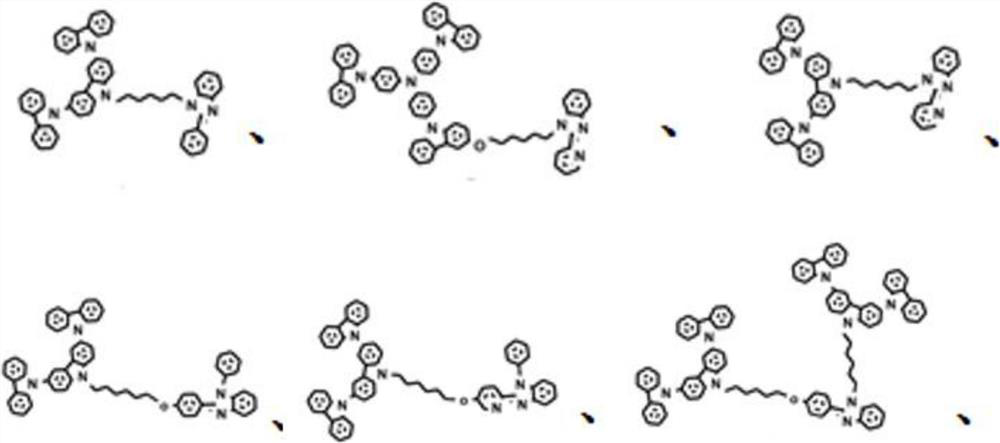
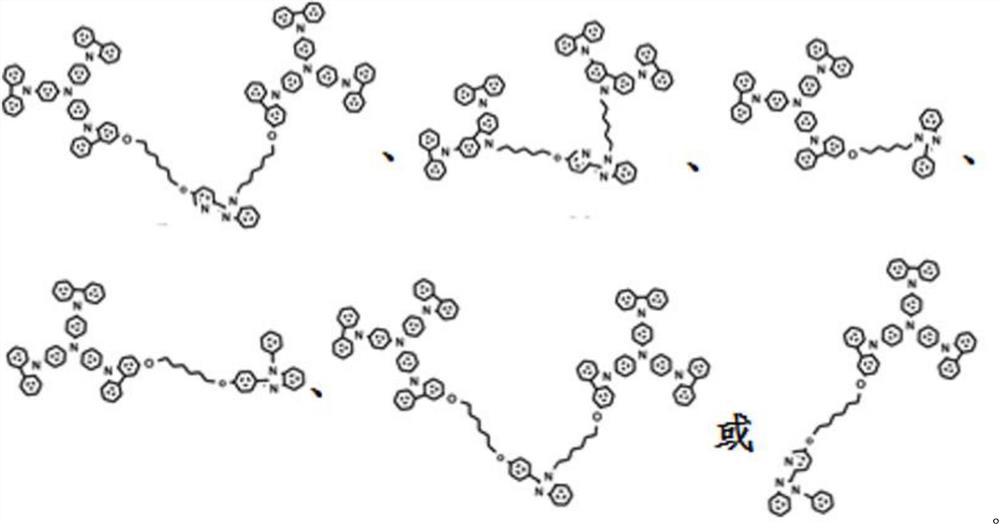
Solution processable dendritic iridium complex electroluminescent material and its synthesis method
A technology of iridium complexes and solution processing, which is applied in the direction of luminescent materials, compounds containing elements of group 8/9/10/18 of the periodic table, chemical instruments and methods, etc., which can solve the problems of slow research progress, luminous brightness and efficiency Low, electroluminescent materials are not easy to purify, etc., to achieve the effect of good repeatability, increased charge transport capacity, and excellent wet film-forming performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1: Synthesis of Ligand L1
[0062] Step 1, the synthesis of 2-phenylbenzimidazole (A1)
[0063] O-phenylenediamine (5.24g, 48mmol) and benzaldehyde (4.37g, 40mmol) were dissolved in DMF respectively, and reacted at room temperature for 8h. After the reaction was completed, the reactant was poured into water to obtain the product A1 with a yield of 80%.
[0064] Mass spectrum: 191.26
[0065] Elemental analysis showed the following results: C: 76.13, H: 5.31, N: 16.99.
[0066] The synthesis process of A1 is shown in the figure below
[0067]
[0068] Step 2, Synthesis of 2I-Cz
[0069] Add carbazole (C Z ) (20g, 119.61mmol), KI (26.21g, 162mmol), KIO 3 (23.38g, 109.04mmol), HAc (340mL), reflux at 120°C for 0.5h. After the reaction is over, rotate steam while it is hot, dissolve the obtained solid with dichloromethane, and wash with saturated saline, saturated NaHCO 3 Aqueous solution, saturated NaHSO 3 Washed with aqueous solution, the organic layer ...
Embodiment 2
[0084] Embodiment 2: Synthesis of L9
[0085] Step 1, synthesis of ligand L9
[0086] The synthesis procedure of ligand L9 is basically the same as that of L1, and the yield is 63%.
[0087]Mass spectrum: 1032.45
[0088] Elemental analysis: C: 84.86, H: 5.46, N: 8.13.
example 3
[0089] Example 3: Synthesis of Ligand L2
[0090] Step 1, the synthesis of 2-phenyl imidazopyridine (A2)
[0091] O-phenylenediamine (5.24g, 48mmol) and pyridinecarbaldehyde (4.37g, 40mmol) were dissolved in DMF respectively, and an aqueous solution of sodium thiosulfate (7.92g, 40mmol) was added under ice-cooling, and heated to 90°C in an oil bath to react overnight. After the reaction was completed, the reactant was poured into water to obtain product A2 with a yield of 70%.
[0092] Mass spectrum: 193.26
[0093] Elemental analysis, the results are as follows: C: 76.00, H: 5.01, N: 18.99.
[0094] The synthesis process of A2 is shown in the figure below
[0095]
[0096] Step 2, Synthesis of 3I-TPA
[0097] Add triarylamine (10g, 40mmol) into a three-necked flask, add potassium iodide (14.36g, 88.81mmol), 150ml of glacial acetic acid, reflux reaction at 120°C, add potassium iodate (9.52g, 44.41mmol) to the above reaction in batches , Reaction 4h. The treatment meth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com