Propane dehydrogenation catalyst and preparation method thereof, and method for preparing propylene by propane dehydrogenation
A propane dehydrogenation and catalyst technology, which is applied in the direction of chemical instruments and methods, physical/chemical process catalysts, hydrocarbons, etc., can solve the problems of unfavorable large-scale production, prone to mistakes or errors, etc., and achieve a favorable ecological environment protection, easy operation, and high propane conversion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 1) Pre-carbonize 10 g of dried waste tea leaves in a nitrogen atmosphere at 400° C. for 2 hours, and cool naturally to room temperature;
[0039] 2) Pulverize the pre-carbonized sample, mix it with sodium hydroxide solution, the mass ratio of sample to sodium hydroxide is 1:1, and evaporate the solution to dryness after stirring for 1 hour;
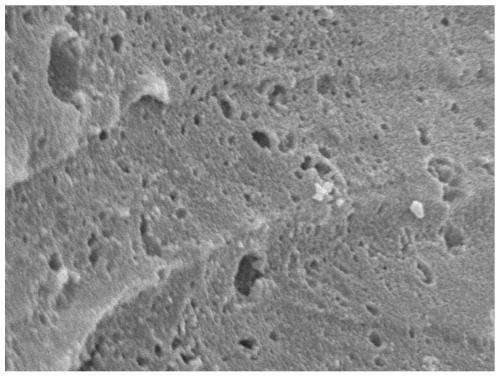
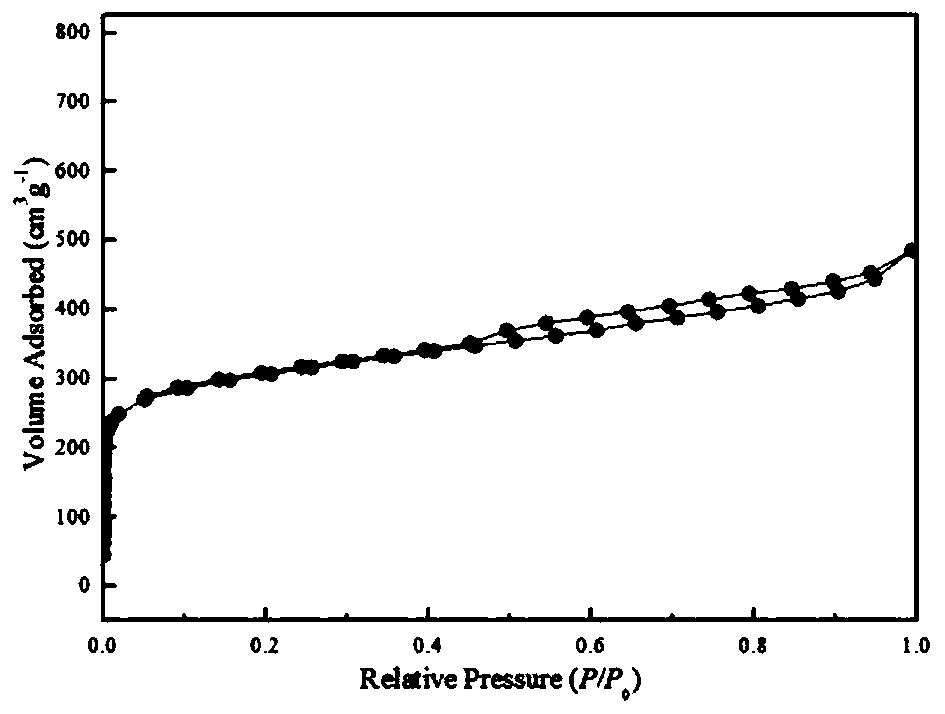
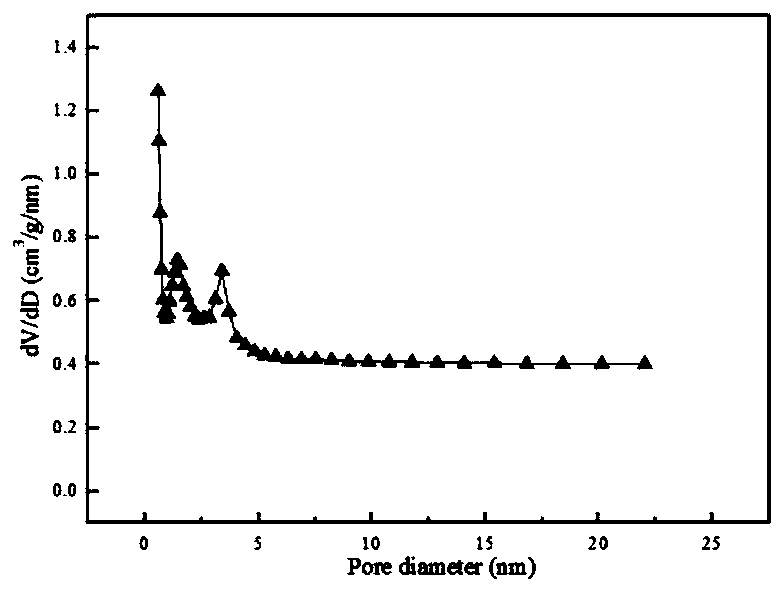
[0040] 3) Activate the obtained product at 700°C under nitrogen for 1 hour, wash until neutral, and dry to obtain a biomass carbon material with a specific surface of 1025m 2 g -1 , the pore volume is 0.75cc / g.
[0041] Evaluation conditions: the amount of catalyst is 0.2g, the mass space velocity of propane is 3000h -1 , the reaction pressure is normal pressure. The temperature of the reaction stability test of the catalyst is 600°C.
[0042] The results show that the initial propane conversion rate of the biomass carbon material catalyst synthesized by the present invention is 65.5%, and the propylene selectivity is 92.2%. Af...
Embodiment 2
[0044] Steps 1) and 2) are the same as in Example 1, the activation temperature in step 3) is increased to 800° C., and other reaction conditions remain unchanged. The obtained biomass carbon material surface is 1058m 2 g -1 , the pore volume is 1.65cc / g.
[0045] The evaluation conditions are the same as in Example 1, and the results show that the initial propane conversion rate of the biomass carbon material catalyst synthesized by the present invention is 39.8%, and the selectivity of propylene is 87.5%. After 50 hours of reaction, the conversion rate of propane is 27%, and the selectivity of propylene is 27%. was 90.4%.
Embodiment 3
[0047] Steps 1) and 3) are the same as in Example 1, the mass ratio of the pre-carbonized sample in step 2) to sodium hydroxide is 1:2, and other reaction conditions remain unchanged. The obtained biomass carbon material surface is 1274m 2 g -1 , the pore volume is 0.98cc / g.
[0048] The evaluation conditions are the same as in Example 1, and the results show that the initial propane conversion rate of the biomass carbon material catalyst synthesized by the present invention is 62.6%, and the propylene selectivity is 92.5%. After 50 hours of reaction, the propane conversion rate is 29.8%, and the propylene selectivity was 93.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com