Method for preparing N,N-Dimethylaniline compound through alkylation of alkyl tosylate
A technology of alkyl toluene sulfonate and dialkylaniline, which is applied in the field of organic synthesis, can solve the problems of highly restricted industrialization development, environmental pollution by bromide ions, high cost, etc. Simple process, the effect of speeding up the rate of formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Add a magnetic stirrer to the 100mL reaction flask, install a thermometer, add 9.5g of p-toluenesulfonyl chloride (0.05mol), dissolve it in 20mL of pyridine, place the reaction flask in an ice-water bath and stir, and add n-hexanol dropwise. The amount of alcohol added is 5.1g (0.05mol), and the reaction time is 6h. Invert the reaction solution in ice water, add concentrated hydrochloric acid until it becomes acidic, extract, and steam distill directly to obtain pure hexyl p-toluenesulfonate.
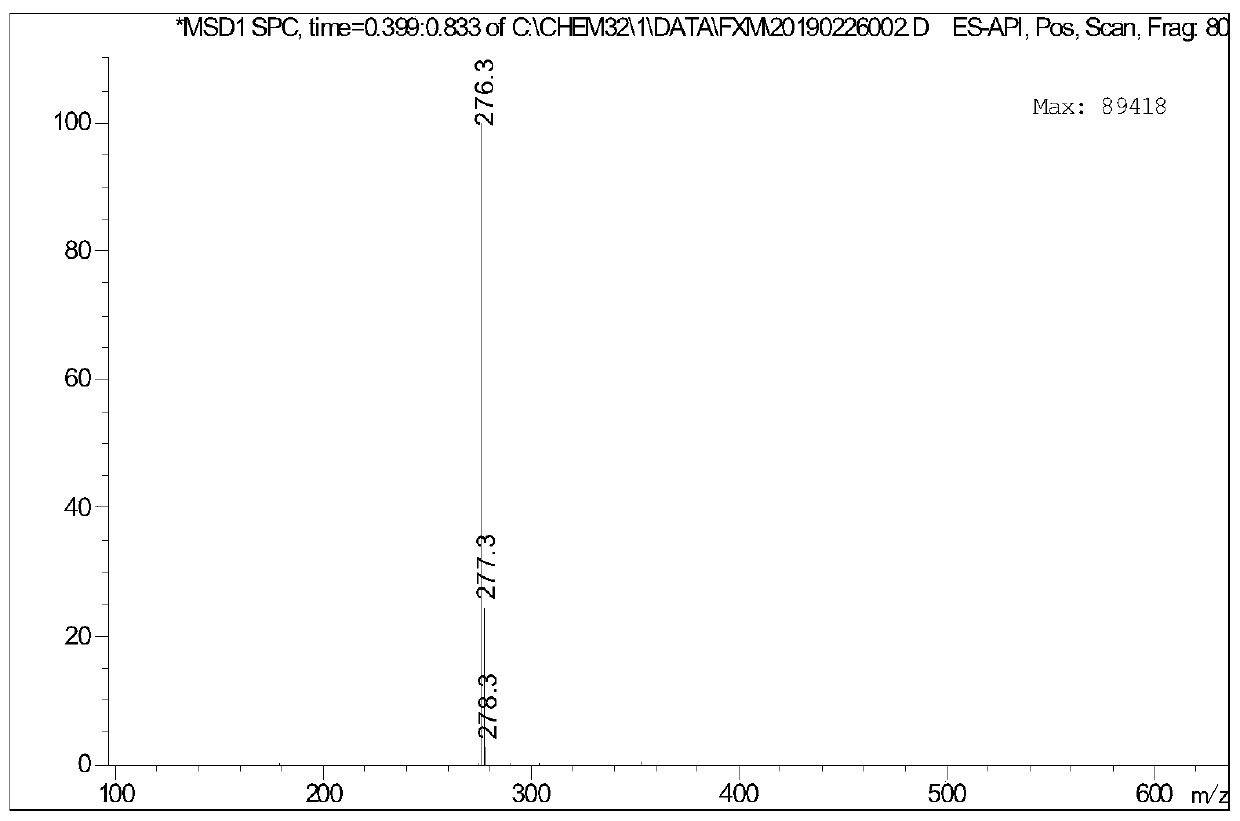
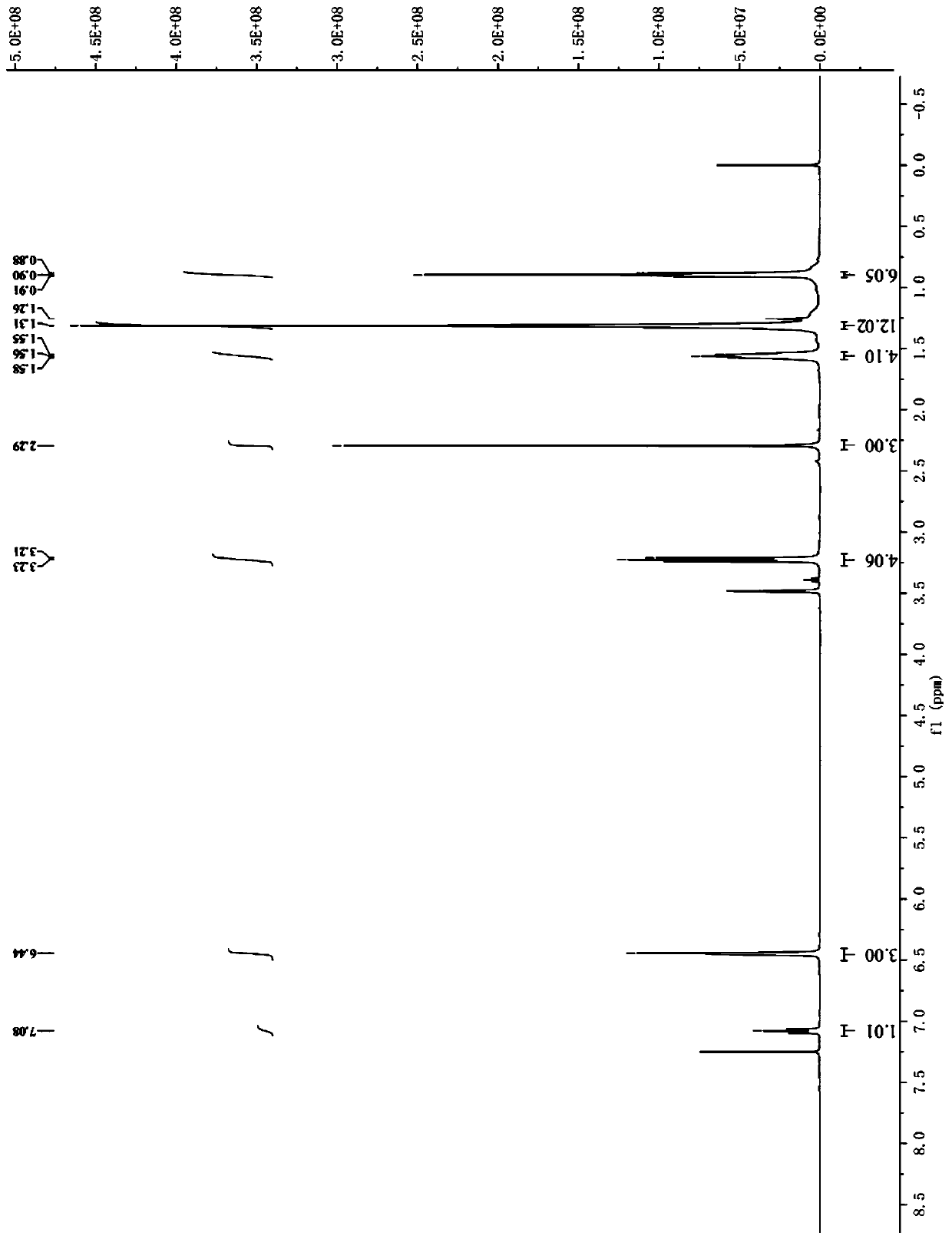
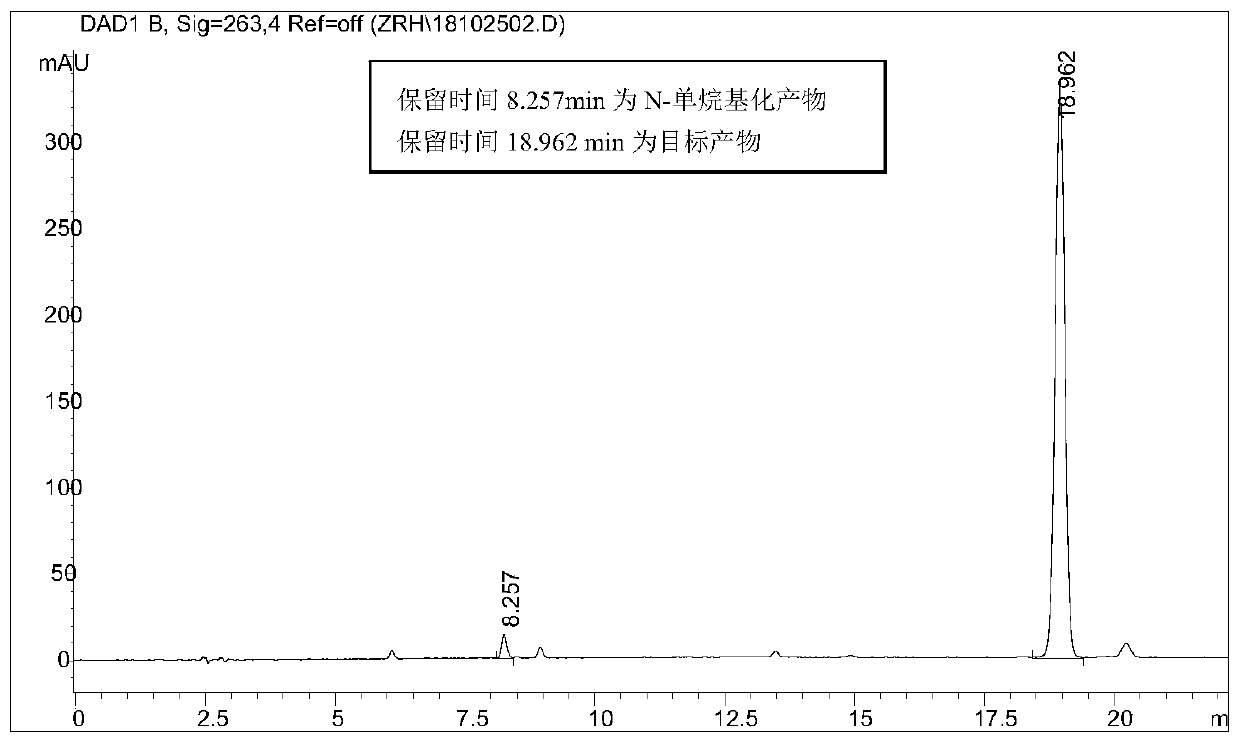
[0066] Take 2.56g (0.01mol) of hexyl p-toluenesulfonate, add 0.62g (0.0036mol) p-bromoaniline at room temperature, add 0.05g tetrabutylammonium bromide phase transfer catalyst, raise the temperature to 100°C and add 25% hydrogen dropwise Sodium oxide solution 1.6g (0.01mol), after keeping the temperature unchanged for 8 hours, lowered to room temperature, extracted with ethyl acetate, dried and filtered, and rotary evaporated to obtain a dark purple viscous liquid, which was separ...
Embodiment 2
[0068] Add a magnetic stirrer to the 100mL reaction flask, install a thermometer, add 9.5g of p-toluenesulfonyl chloride (0.05mol), dissolve it in 20mL of pyridine, place the reaction flask in an ice-water bath and stir, and add n-hexanol dropwise. The amount of alcohol added is 5.1g (0.05mol), and the reaction time is 6h. Invert the reaction solution in ice water, add concentrated hydrochloric acid until it becomes acidic, extract, and steam distill directly to obtain pure hexyl p-toluenesulfonate.
[0069] Take 2.56g (0.01mol) of hexyl p-toluenesulfonate, add 0.46g (0.0036mol) of p-chloroaniline at room temperature, add 0.05g of tetrabutylammonium bromide phase transfer catalyst, raise the temperature to 100°C and add 25% hydrogen dropwise Sodium oxide solution 1.6g (0.01mol), keep the temperature constant and react for 8 hours, then cool down to room temperature, extract with ethyl acetate, dry and filter, and rotary evaporate to obtain a dark purple viscous liquid, which is...
Embodiment 3
[0071] Add a magnetic stirrer to the 100mL reaction flask, install a thermometer, add 9.5g of p-toluenesulfonyl chloride (0.05mol), dissolve it in 20mL of pyridine, place the reaction flask in an ice-water bath and stir, and add n-hexanol dropwise. The amount of alcohol added is 5.1g (0.05mol), and the reaction time is 6h. Invert the reaction solution in ice water, add concentrated hydrochloric acid until it becomes acidic, extract, and steam distill directly to obtain pure hexyl p-toluenesulfonate.
[0072] Take 2.56g (0.01mol) of hexyl p-toluenesulfonate, add 0.38g (0.0036mol) p-toluidine at room temperature, add 0.05g tetrabutylammonium bromide phase transfer catalyst, raise the temperature to 100°C and add 25% hydrogen dropwise Sodium oxide solution 1.6g (0.01mol), keep the temperature constant and react for 8 hours, then cool down to room temperature, extract with ethyl acetate, dry and filter, and rotary evaporate to obtain a dark purple viscous liquid, which is separated...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com