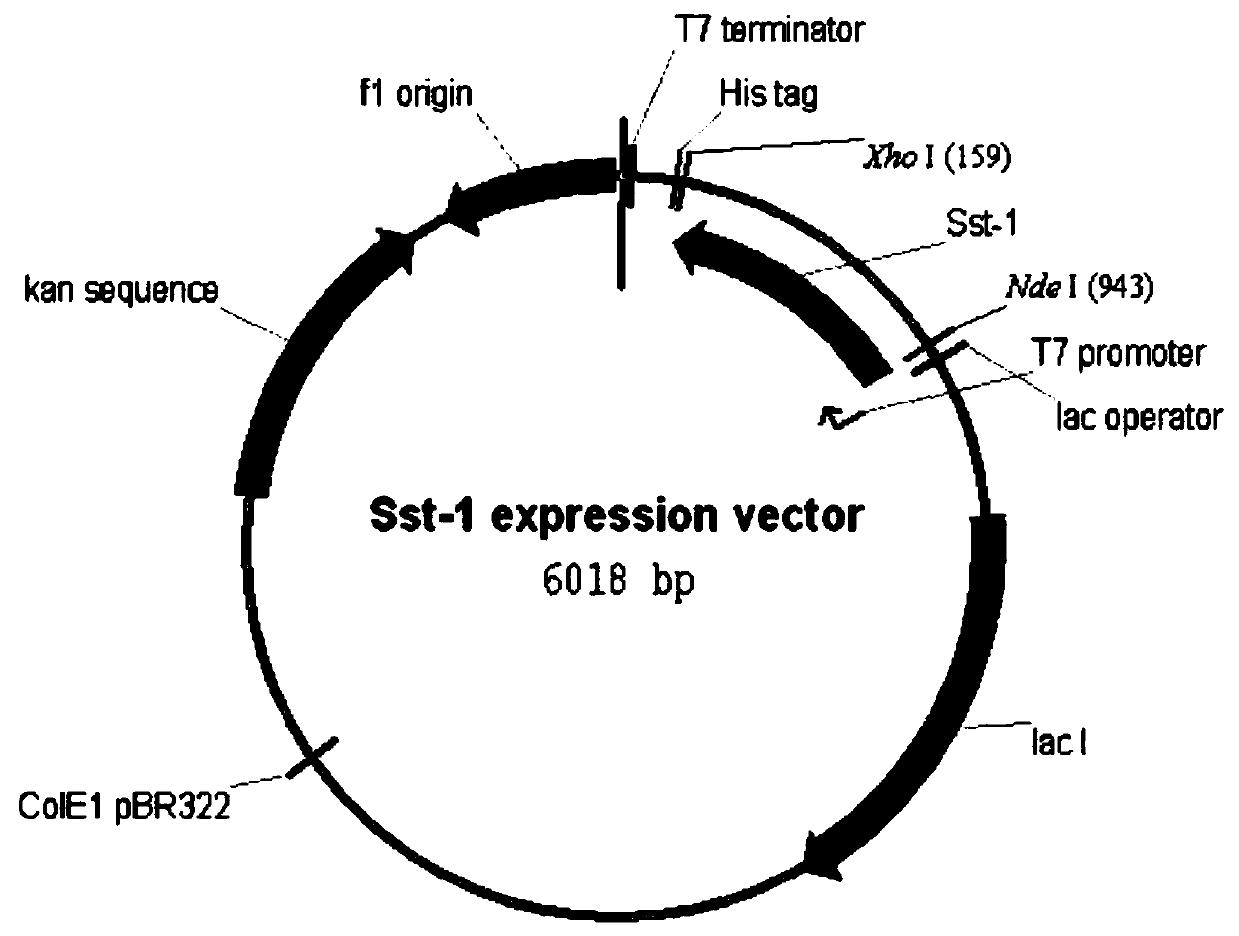
Bioconversion method for anti-AIDS-medicine-reyataz midbody
A biotransformation and anti-AIDS technology, applied in the field of medicine and biology, can solve the problems of too many yeast cells, low enzyme activity, and a large proportion of NAD cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0118] The biotransformation method of the anti-AIDS drug atazanavir intermediate provided in the present embodiment 1 comprises the following steps:
[0119] Through the method of whole gene synthesis, the secondary structure and codon bias of the gene are adjusted to achieve high expression in Escherichia coli.
[0120] Use Primer Premier (http: / / primer3.ut.ee / ) and OPTIMIZER (http: / / genomes.urv.es / OPTIMIZER / ) to design, and ensure that the Tm difference is controlled within 3°C, and the primer length is controlled within 60base , resulting in the following primers:
[0121] 1 TGTTTAACTTTAAGAAGGAGATATACATATGACCATCG
[0122] CCGGTAACAACAGCAACAACGTTGTTCAGAGCGATGGTCATA
[0123] 2 TGTATATCTCCCTT
[0124] GTTGTTGCTGTTGTTACCGGTGCTGCTGGTGGTATCGGTCGTG
[0125] 3 AACTGGTTAAAG
[0126] GCAGCGATAACGATAGCGTTAGCAGCTTTCATAGCTTTAACCA
[0127] 4 GTTCACGACCGA
[0128] ACGCTATCGTTATCGCTGCTGAAATGGCTCCGTCTGCTGACAA
[0129] 5 AGAAGGTGCTGA
[0130] CAGCTTCAGAGGTAACGTCGTGCTGCAGGTAGTGGTCAG...
Embodiment 2
[0172] The biotransformation method of the anti-AIDS drug atazanavir intermediate provided in this embodiment 2 comprises the following steps:
[0173] Through the method of whole gene synthesis, the secondary structure and codon bias of the gene are adjusted to achieve high expression in Escherichia coli.
[0174] Use Primer Premier (http: / / primer3.ut.ee / ) and OPTIMIZER (http: / / genomes.urv.es / OPTIMIZER / ) to design, and ensure that the Tm difference is controlled within 3°C, and the primer length is controlled within 60base , resulting in the following primers:
[0175] 1 TGTTTAACTTTAAGAAGGAGATATACATATGACCATCG
[0176] CCGGTAACAACAGCAACAACGTTGTTCAGAGCGATGGTCATA
[0177] 2 TGTATATCTCCCTT
[0178] GTTGTTGCTGTTGTTACCGGTGCTGCTGGTGGTATCGGTCGTG
[0179] 3 AACTGGTTAAAG
[0180] GCAGCGATAACGATAGCGTTAGCAGCTTTCATAGCTTTAACCA
[0181] 4 GTTCACGACCGA
[0182] ACGCTATCGTTATCGCTGCTGAAATGGCTAAATCTGCTGACAA
[0183] 5 AGAAGGTGCTGA
[0184] CAGCTTCAGAGGTAACGTCGTGCTGCAGGTAGTGGTCAGCA
[01...
Embodiment 3
[0226] The biotransformation method of the anti-AIDS drug atazanavir intermediate provided in this embodiment 3 comprises the following steps:
[0227] Through the method of whole gene synthesis, the secondary structure and codon bias of the gene are adjusted to achieve high expression in Escherichia coli.
[0228] Use Primer Premier (http: / / primer3.ut.ee / ) and OPTIMIZER (http: / / genomes.urv.es / OPTIMIZER / ) to design, and ensure that the Tm difference is controlled within 3°C, and the primer length is controlled within 60base , resulting in the following primers:
[0229] 1 TGTTTAACTTTAAGAAGGAGATATACATATGACCATCGC
[0230] ACCGGTAACAACAGCAACAACGTTGTTCAGAGCGATGGTCAT
[0231] 2 ATGTATATCTCCT
[0232] TTGTTGCTGTTGTTACCGGTGCTGCTGGTGGTATCGGTCGTGA
[0233] 3 ACTGGTTAAAGC
[0234] CAGCAGCGATAACGATAGCGTTAGCAGCTTTCATAGCTTTAAC
[0235] 4 CAGTTCACGACC
[0236] CGCTATCGTTATCGCTGCTGAAATGGCTAAATCTGCTAACAAA
[0237] 5GAAGGTGCTGAC
[0238] CAGCTTCAGAGGTAACGTCGTGCTGCAGGTAGTGGTCAGCA
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com