Synthesis method of natural product (-)-newbouldine
A synthetic method and technology of natural products, applied in the direction of organic chemistry, etc., can solve the problems of harsh reaction conditions, low yield, difficult post-processing, etc., and achieve the effects of mild reaction conditions, easy post-processing, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025]
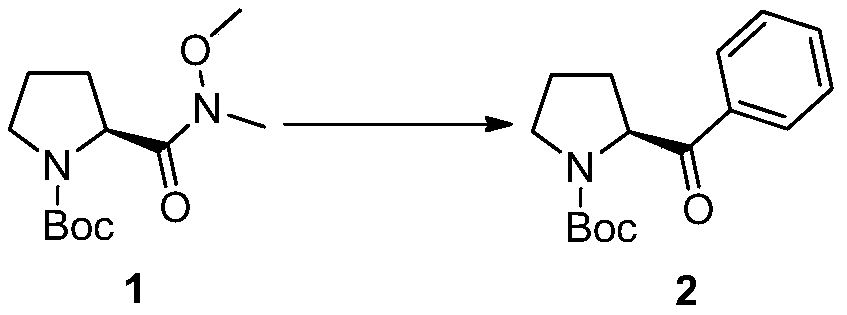
[0026] 1. Under the protection of argon, dissolve 2.6g (10mmol) of compound 1 in redistilled tetrahydrofuran, add 14mL of 1mol / L diethyl ether solution of phenylmagnesium bromide dropwise at 0°C, and stir for 30 minutes after the dropwise addition , and then the reaction was quenched by adding saturated aqueous ammonium chloride. The resulting mixture was extracted 3 times with ether, washed 3 times with saturated brine, dried over anhydrous sodium sulfate, concentrated with a rotary evaporator, and finally subjected to column chromatography (ethyl acetate:petroleum ether=5:100~20:100, v / v) Purification to obtain 1.72g of white solid compound 2, whose chemical name is tert-butyl (S)-2-benzoylpyrrole-1-carbonate, the yield is 62%, and the structural characterization data are as follows: 1 H NMR (600MHz, CDCl 3 )δ7.97(dd, J=21.2,7.4Hz,1.97H),7.57(dt,J=21.2,7.4Hz,1H),7.47(dt,J=20.0,7.7Hz,2H),5.37-5.31( m,0.39H),5.20(dd,J=8.8,3.8Hz,0.59H),3.72-3.61(m,1H),3.56(dt,J=1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com