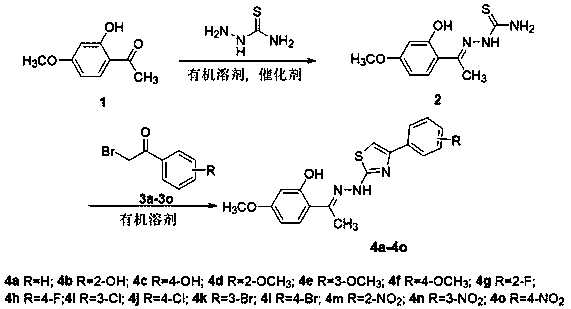
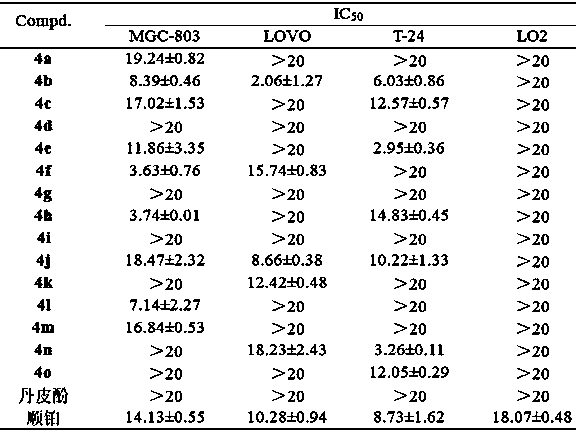
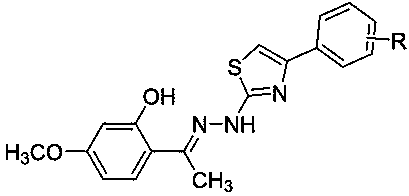
Paeonol thiazole derivative and preparation method and application thereof
A technology of paeony phenothiazole derivatives, which is applied in the field of paeony phenothiazole derivatives and its preparation, can solve the problems that there are no public reports on paeony phenothiazole derivatives, and achieve improved anti-tumor activity, stable quality, The effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the preparation of paeonol thiosemicarbazone 2
[0032] Add 33mmol of thiosemicarbazide and 40mL of absolute ethanol to a 250mL dry round-bottom flask, heat, stir and reflux at 80°C for 30min, slowly add 60mL of hot paeonol (30mmol) in absolute ethanol In the round bottom flask, add the concentrated sulfuric acid of 1mL again, continue to heat and stir reaction, TLC tracks reaction progress (V 氯仿 :V 甲醇 =10:1), stop the reaction after 20 h of reaction, add 80 mL of ice water, filter and wash to obtain 5.4 g of light yellow solid with a yield of 75.2%; m.p. 187.2-189.0°C; IR (KBr, cm -1 ) : 3562 (OH), 3379 (NH 2 ), 3271 (NH), 3138, 2976, 2841, 1624(C=N), 1589, 1514, 1479, 1340, 1253, 1166, 1080, 970, 837, 792, 744; MS (ESI)m / z 240.0 ([M+H] + );
[0033] Therefore, above-mentioned compound 2 is paeonol thiosemicarbazone, and its structural formula is as follows:
[0034] .
Embodiment 2
[0035] Example 2: N Preparation of -(4-phenylthiazol-2-yl)-2-hydroxy-4-methoxyacetophenone hydrazone 4a
[0036] Add 1 mmol paeonol thiosemicarbazone and 1 mmol compound 3a in a 100 mL round bottom flask, add 20-80 mL absolute ethanol, stir and reflux at 80 °C, and monitor the reaction progress by TLC (V 石油醚 :V 乙酸乙酯 =3:1). After the reaction, cool, filter, wash the precipitate with absolute ethanol, dry, and recrystallize with absolute ethanol and chloroform to obtain the solid product 4a, a light yellow solid, with a yield of 73.0%; m.p. 195.0-196.1 ℃; IR (KBr, cm -1 ): 3412 (OH), 3232 (NH), 3049, 2931,2849, 1620 (C=N), 1591, 1510, 1454, 1363, 1282, 1199, 1155, 1111, 1025, 975,858, 754, 671; 1 H NMR (600 MHz, DMSO) δ 7.86 (d, J = 7.5 Hz, 2H, H-2’, H-6’),7.50 (d, J = 8.5 Hz, 1H, H-4’), 7.42 (t, J = 7.2 Hz, 2H, H-3’, H-5’), 7.32(t, J = 7.1 Hz, 1H, H-6’), 7.28 (s, 1H, H-10), 6.49 (d, J = 15.1 Hz, 2H, H-5,H-3), 3.76 (s, 3H, OCH 3 ), 2.40 (s, 3H, CH 3 ); 13 C NMR...
Embodiment 3
[0039] Example 3:N Preparation of -[4-(2-hydroxyphenyl)thiazol-2-yl]-2-hydroxy-4-methoxyacetophenone hydrazone 4b
[0040] In a 100 mL round bottom flask, add 1 mmol paeonol thiosemicarbazone and 1 mmol compound 3b, add 20-80 mL absolute ethanol, stir and reflux reaction at 80 ° C, TLC monitors the reaction process (V 石油醚 :V 乙酸乙酯 =3:1). After the reaction, cool, filter, wash the precipitate with absolute ethanol, dry, and recrystallize with absolute ethanol and chloroform to obtain a yellow solid 4b with a yield of 82.3%; m.p.231.0-234.0 ℃; IR (KBr, cm -1 ) : 3414 (OH), 3097, 2937, 2841, 1620 (C=N),1516, 1458, 1361, 1284, 1193, 1159, 1107, 1028, 976, 858, 743; 1 H NMR (600MHz, DMSO) δ 8.04 (t, J = 7.7, 1.3 Hz, 1H, H-6'), 7.50 (d, J = 8.7 Hz, 1 H-6), 7.40-7.36 (m, 1H, H-4'), 7.33-7.27 (m, 2H, H-5', H-10), 7.26 (d, J = 2.1Hz, 1H, H-3'), 6.50 (dd, J = 8.7, 2.6 Hz, 1H, H-5), 6.48 (d, J = 2.5 Hz, 1H, H-3), 3.76 (s, 3H, OCH 3 ), 2.39 (s, 3H, CH 3 ); 13 C NMR (151 mHz, DMSO) Δ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com