Fluorescent probe having hydrogen peroxide detection function and preparation method and application thereof
A fluorescent probe and reaction technology, applied in chemical instruments and methods, organic chemistry, fluorescence/phosphorescence, etc., can solve the problems of complex synthesis route, enzymatic decomposition, low content, etc., and achieve simple preparation route, low synthesis cost, processing handy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
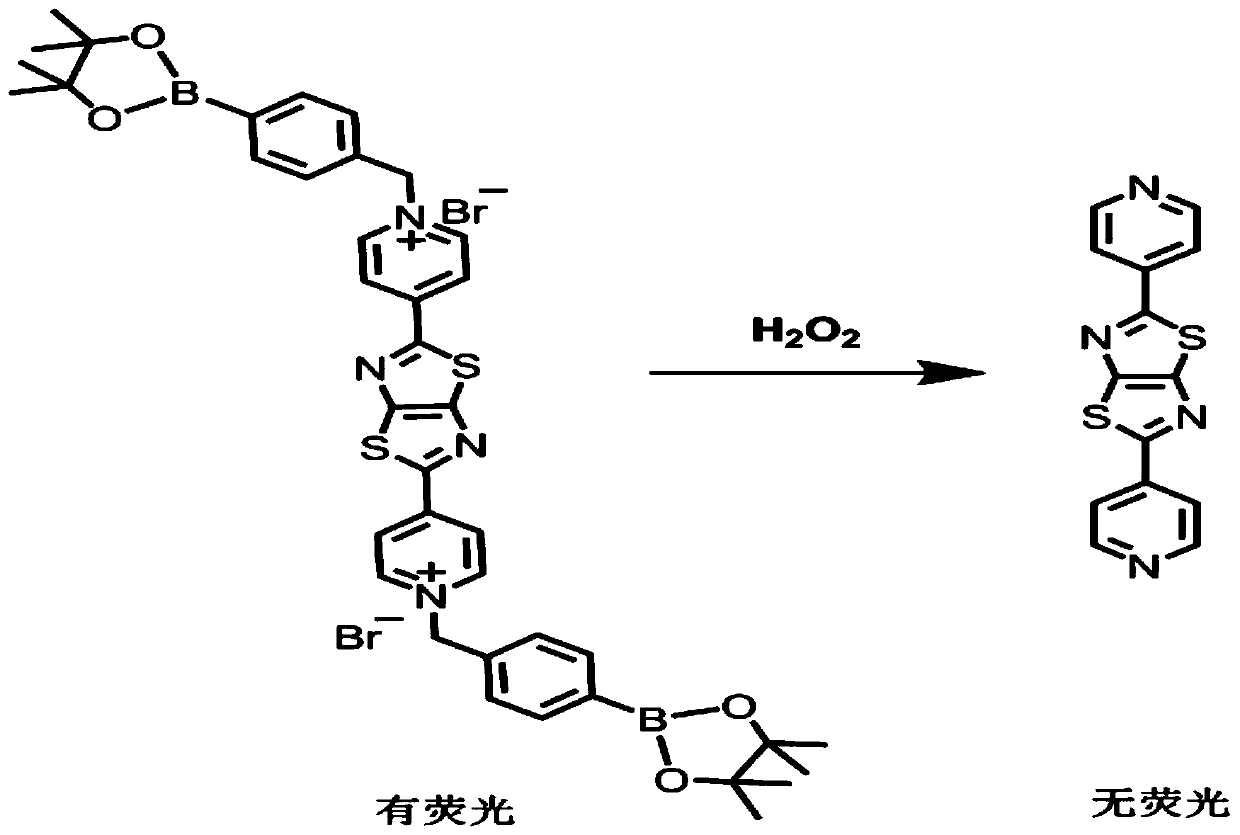
[0027] One capable of detecting H 2 o 2 The preparation method of fluorescent probe comprises the following steps:
[0028] (1) Preparation of 2,5-bis(4-pyridyl)thiazolo[5,4-d]thiazole: Dithiooxamide (1mmol) and 4 -Pyridinecarbaldehyde (3mmol) was dissolved in 10mL of anhydrous N,N'-dimethylformamide, heated to reflux at 150°C, reacted for 8h, filtered, then rinsed with deionized water, and dried in vacuo to obtain 2,5-bis (4-pyridyl)thiazolo[5,4-d]thiazole.
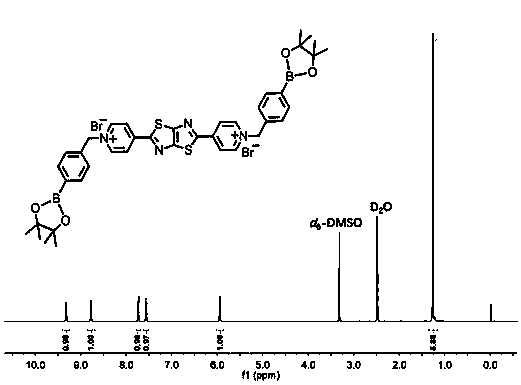
[0029] (2) Preparation of fluorescent probe N,N'-bis(4-methylenemethylphenylboronic acid) 2,5-bis(4-pyridinium salt)thiazolo[5,4-d]thiazole dibromide: 2 , 5-bis(4-pyridyl)thiazolo[5,4-d]thiazole (0.1mmol) and 4-bromomethylphenylboronic acid pinacol ester (7mmol) were dissolved in 10mL of acetonitrile and heated to 90°C for reaction 6h, filtered, rinsed with n-hexane, and dried under vacuum to obtain the small molecule fluorescent probe N,N'-bis(4-methylenemethylphenylboronic acid) 2,5-bis(4-pyridinium salt)thiazolo[5...
Embodiment 2
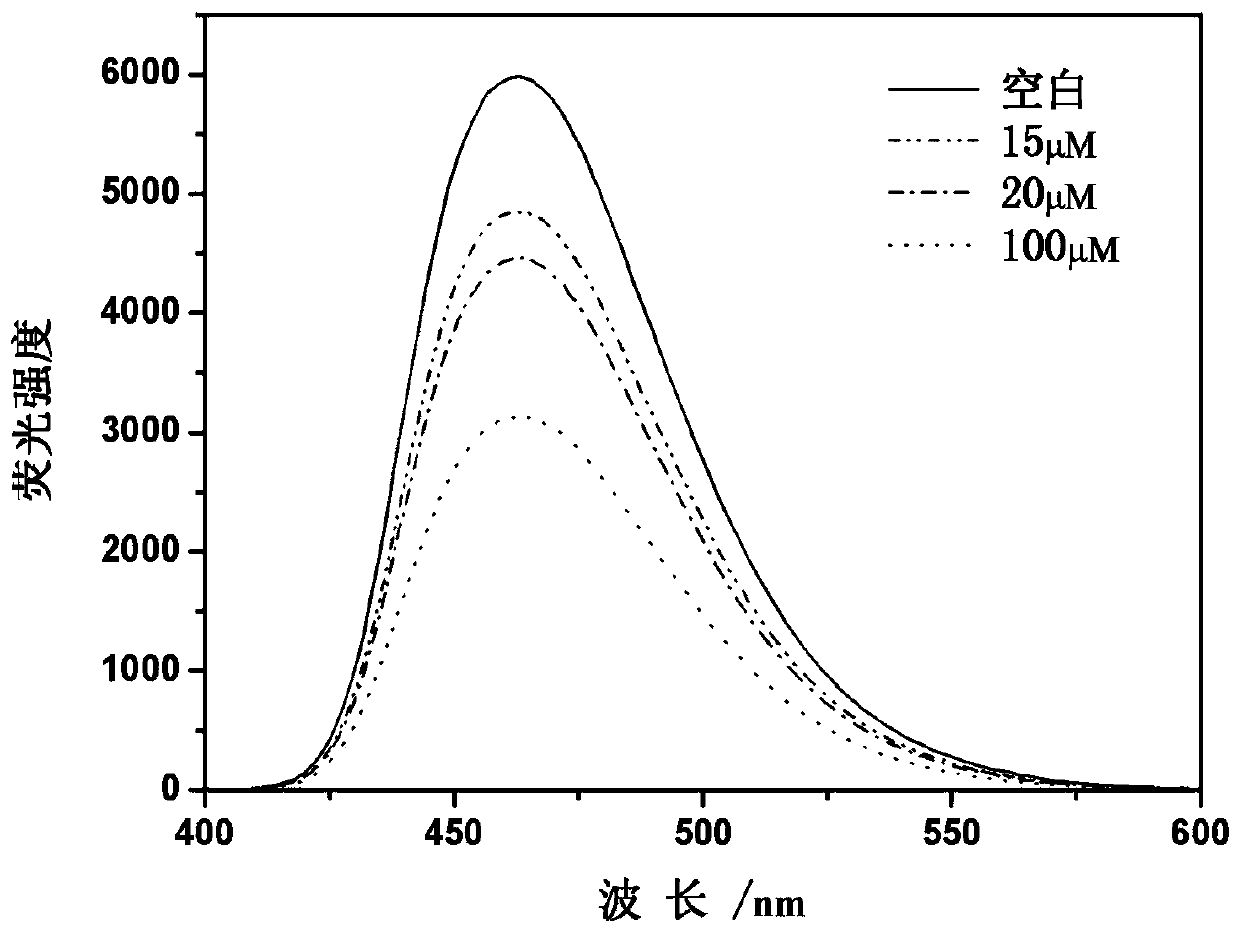
[0031] Prepare 11 parts of fluorescent probe solution with distilled water, the solution concentration is 2.5μM, the volume is 3mL, and then, the H 2 o 2 0 μM (a), 15 μM (b), 20 μM (c), 100 μM (d) in 30 μL of HO 2 o 2 Solution is added in every part of probe solution, after stirring at room temperature for 30S, measure the fluorescence intensity of every part of solution, 395nm is used as excitation wavelength, then obtain the fluorescence emission spectrum change pattern of every part of solution ( image 3 ). The test results show that with the H 2 o 2 As the concentration increases, the fluorescence intensity of the fluorescent probe decreases. and H 2 o 2 When the concentration is greater than 20 μM, the fluorescence intensity decreases significantly.
Embodiment 3
[0033] Prepare 1 part of the fluorescent probe solution with distilled water, the solution concentration is 2.5 μM, the volume is 3 mL, and then add 100 μM (d) 30 μL of H 2 o 2 solution into the probe solution, after stirring at room temperature, measure the fluorescence intensity of the solution along with the reaction time, 395nm is used as the excitation wavelength, then obtain the fluorescence emission spectrum change spectrum of the solution ( Figure 4 ). The test results show that with the increase of the reaction time, the fluorescence intensity of the fluorescent probe decreases, and when the reaction time is 3 minutes, the fluorescence intensity decreases obviously.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com