Preparation and quality control method of white paeony root formula granule reference substance
A quality control method and technology of formula granules, which are applied in pharmaceutical formulas, medical preparations containing active ingredients, measuring devices, etc., to achieve the effects of quality control, easy storage, and quality assurance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation of Radix Paeoniae Alba Reference Material Formula Granules:
[0028] (1) Weigh raw materials: Radix Paeoniae Alba decoction pieces 6666g;
[0029] (2) Put the decoction pieces of Radix Paeoniae Alba into the decoction pot, decoct twice, add water 9 times the amount of decoction pieces for the first time, soak for 30 minutes, decoct for 30 minutes, add 7 times the amount of decoction pieces for the second time, decoct for 30 minutes Minutes, the liquid medicine is filtered, the filtrates are combined, pumped into the concentration tank, concentrated (vacuum degree -0.090~-0.099MPa), concentrated to a clear paste with a relative density of 1.07-1.09 (65°C), and set aside;
[0030] (3) Take the clear paste obtained in step 2, place the obtained clear paste in a stainless steel plate of a certain specification, place it in a freeze dryer, close the door panel and turn on the refrigeration, wait for the temperature of the partition to drop to the target temperatu...
Embodiment 2
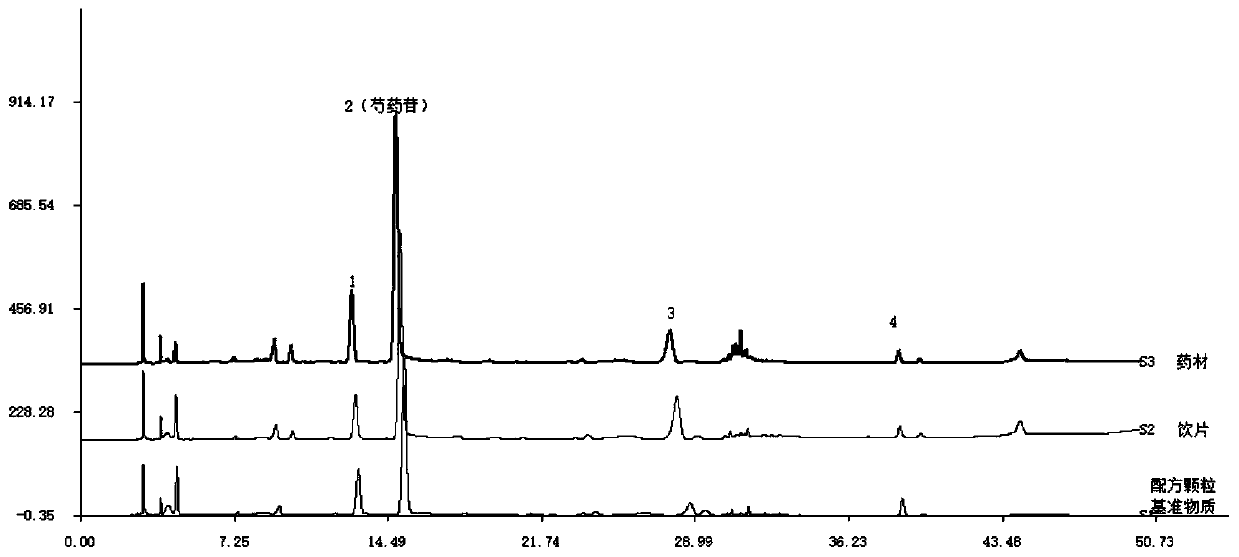
[0036] The quality control method of the HPLC characteristic spectrum of the reference substance of Baishao Formula Granules:
[0037] (1) Take 0.1g of the powder of this product, weigh it accurately, put it in a 25ml measuring bottle, add methanol and sonicate for 30 minutes; let it stand, filter, and filter with a microporous membrane (0.45µm in pore size), and the filtrate is used as the test sample.
[0038] (2) Take 0.6mg of paeoniflorin, put it in a 10ml volumetric flask, dissolve it with methanol and set the volume to the mark, as a reference;
[0039] (3) Refer to the "Chinese Pharmacopoeia" 2015 edition of the four general rules 0512 high-performance liquid chromatography test, respectively accurately absorb 5-10 µl of the test solution and the control solution, inject it into the chromatograph, and measure it according to the following chromatographic conditions;
[0040] Octadecylsilane bonded silica gel is used as filler; the mobile phase is eluted according to the...
Embodiment 3
[0046] Determination of moisture, extracts and content in the reference material of Baishao Formula Granules:
[0047] The moisture, extracts and content determination in the reference material of 3 batches of Radix Paeoniae Alba Formula Granules prepared by the above method were tested. Moisture was detected by conventional methods. The extracts were determined according to the hot soak method under the alcohol-soluble extracts determination method ("Chinese Pharmacopoeia" 2015 Edition Four General Rules 2201), using ethanol as a solvent. The determination of the content is based on the determination method under the item of Radix Paeoniae Alba ("Chinese Pharmacopoeia" 2015 edition one). The inspection results are shown in Table 3, indicating that all indicators meet the standards.
[0048] Table 3 Various test results
[0049] batch number moisture Leachate Paeoniflorin content (%) 1 4.8 54.05 11.45 2 4.2 49.63 8.85 3 4.3 39.37 12.35 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com