Shikonin oxime derivative containing aza-side chain, preparation method and medical application thereof
A derivative, shikonin technology, applied in the field of medicine, can solve the problems of great influence on anti-tumor activity and reduced anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065]
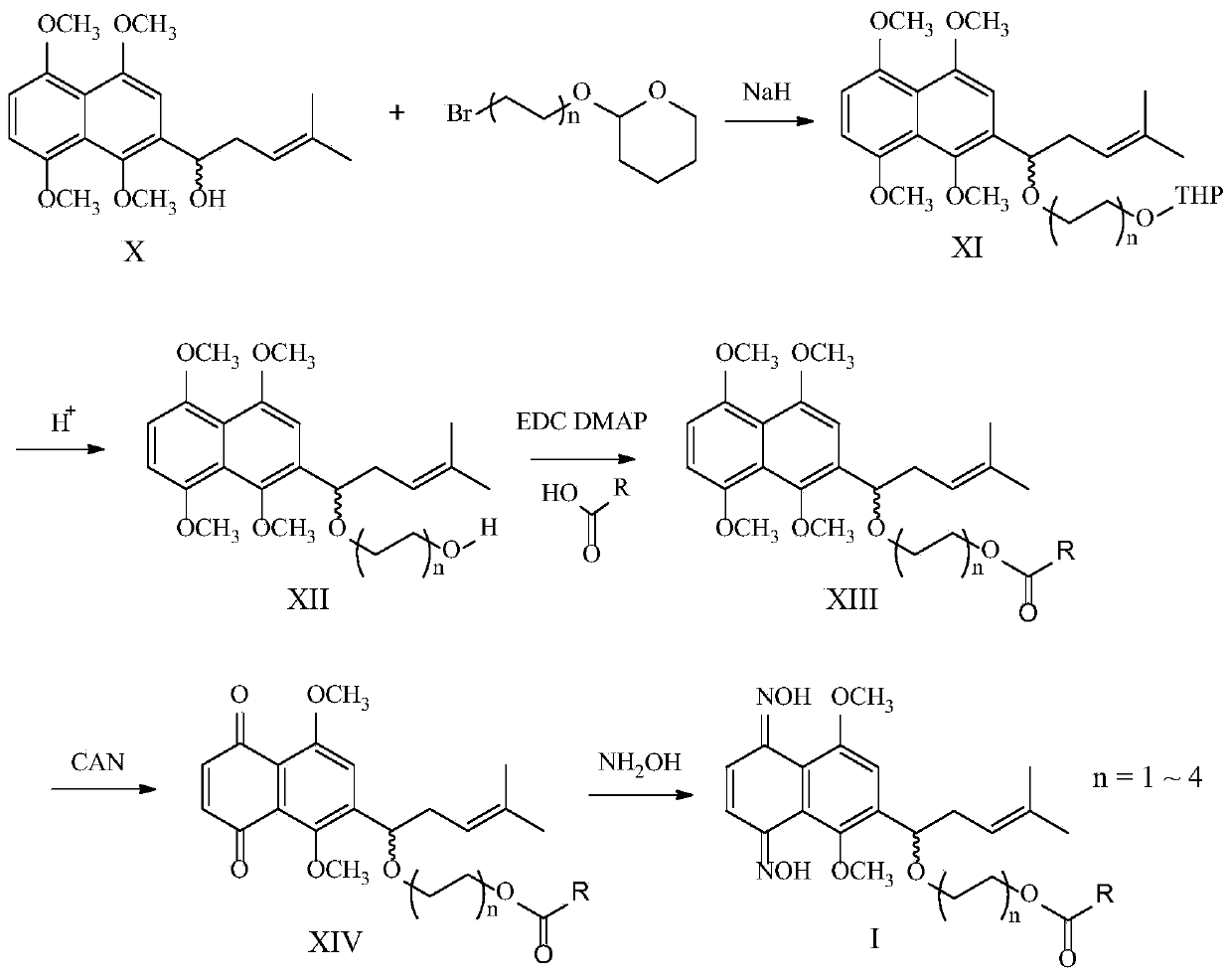
[0066] This example relates to a 2-[1-(8-hydroxyoctyloxy)-4-methyl-3-pentenyl]-1,4,5,8-tetra The preparation method of methoxynaphthalene, such as figure 1 shown, including the following steps:
[0067] Dissolve 2-(1-hydroxy-4-methyl-3-pentenyl)-1,4,5,8-tetramethoxynaphthalene (X) in anhydrous N,N'-dimethylformamide , cooled to 0°C, added 2 times the equivalent of sodium hydride, stirred for 30 minutes, then added 2 times the equivalent of 2-(8-bromooctyloxy)-tetrahydropyran, the reaction solution was heated to 50°C and stirred for 24 Hour. The reaction was quenched by adding ice water, extracted with ethyl acetate, and a light brown oil was obtained after silica gel column chromatography. The oil was dissolved in methanol, 1 ml of concentrated hydrochloric acid was added dropwise, and stirred overnight at room temperature. The reaction solution was concentrated to a small volume under reduced pressure, and extracted with ethyl acetate. The organic layer was w...
Embodiment 2
[0069]
[0070] This embodiment relates to a kind of (R)-2-[1-(8-hydroxyoctyloxy)-4-methyl-3-pentenyl]-1 having the above structural formula ((R)-XII-1) , the preparation method of 4,5,8-tetramethoxynaphthalene, such as figure 1 shown, including the following steps:
[0071] The steps of this example are the same as those of Example 1. In step one, (R)-2-(1-hydroxy-4-methyl-3-pentenyl)-1,4,5,8-tetramethoxy Naphthalene ((R)-X) instead of 2-(1-hydroxy-4-methyl-3-pentenyl)-1,4,5,8-tetramethoxynaphthalene (X). The product was brown oil with a yield of 71%. 1 H NMR (400MHz, CDCl 3 )δ6.95(s,1H),6.76–6.66(m,2H),5.21–5.13(m,1H),4.87–4.76(m,1H),3.88(s,3H),3.71(s,3H) ,3.79(s,3H),3.65(s,3H),3.49–3.41(m,2H),3.28–3.16(m,2H),2.82–2.75(m,1H),2.48–2.33(m,2H) ,1.56(s,3H),1.45(s,3H),1.46–1.34(m,2H),1.30–1.10(m,10H).
Embodiment 3
[0073]
[0074] This example relates to a (S)-2-[1-(8-hydroxyoctyloxy)-4-methyl-3-pentenyl]-1 having the above structural formula ((S)-XII-1) , the preparation method of 4,5,8-tetramethoxynaphthalene, such as figure 1 shown, including the following steps:
[0075] The steps of this example are the same as those of Example 1. In step one, (S)-2-(1-hydroxy-4-methyl-3-pentenyl)-1,4,5,8-tetramethoxy 2-(1-Hydroxy-4-methyl-3-pentenyl)-1,4,5,8-tetramethoxynaphthalene (X) is replaced by substituted naphthalene ((S)-X). The product was brown oil with a yield of 75%. 1 H NMR (400MHz, CDCl 3 )δ6.91(s,1H),6.76–6.66(m,2H),5.21–5.13(m,1H),4.85–4.76(m,1H),3.89(s,3H),3.71(s,3H) ,3.79(s,3H),3.65(s,3H),3.49–3.41(m,2H),3.26–3.16(m,2H),2.81–2.75(m,1H),2.48–2.33(m,2H) ,1.56(s,3H),1.45(s,3H),1.45–1.34(m,2H),1.28–1.10(m,10H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com