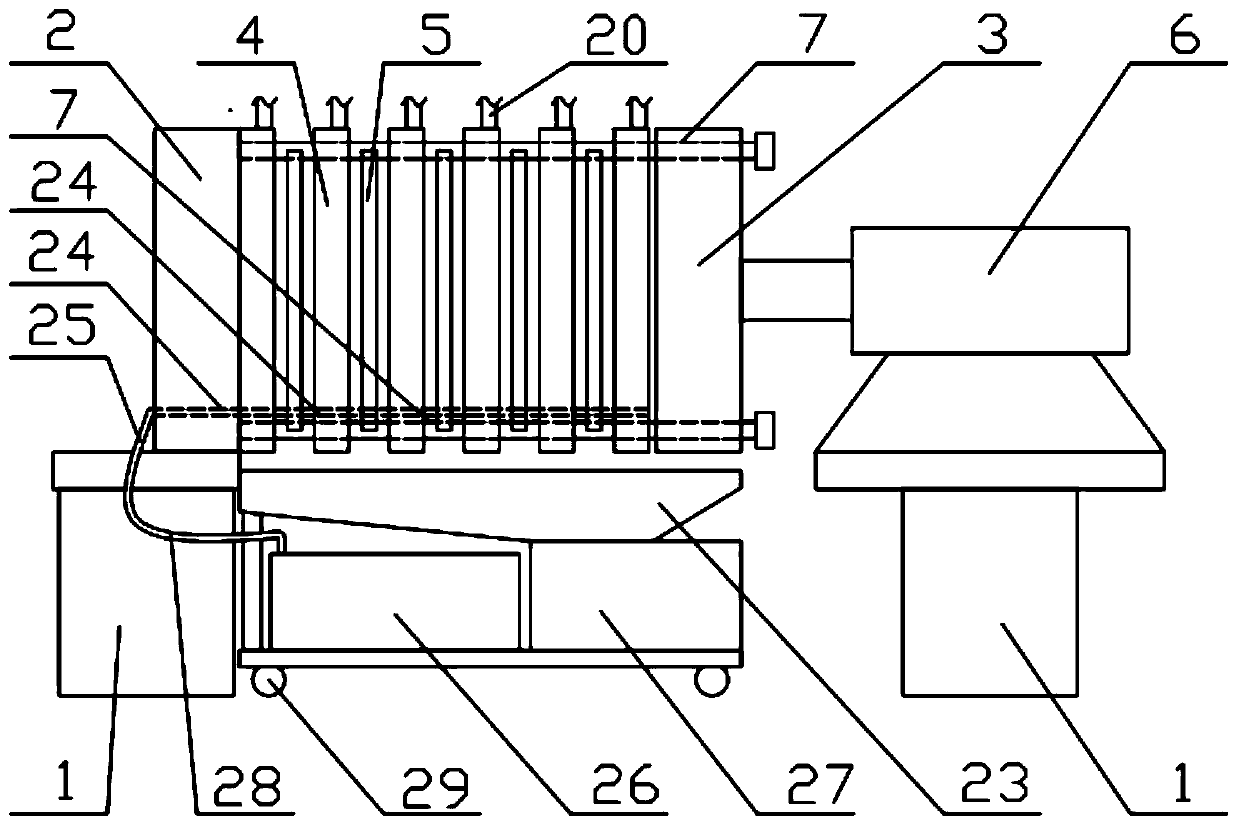
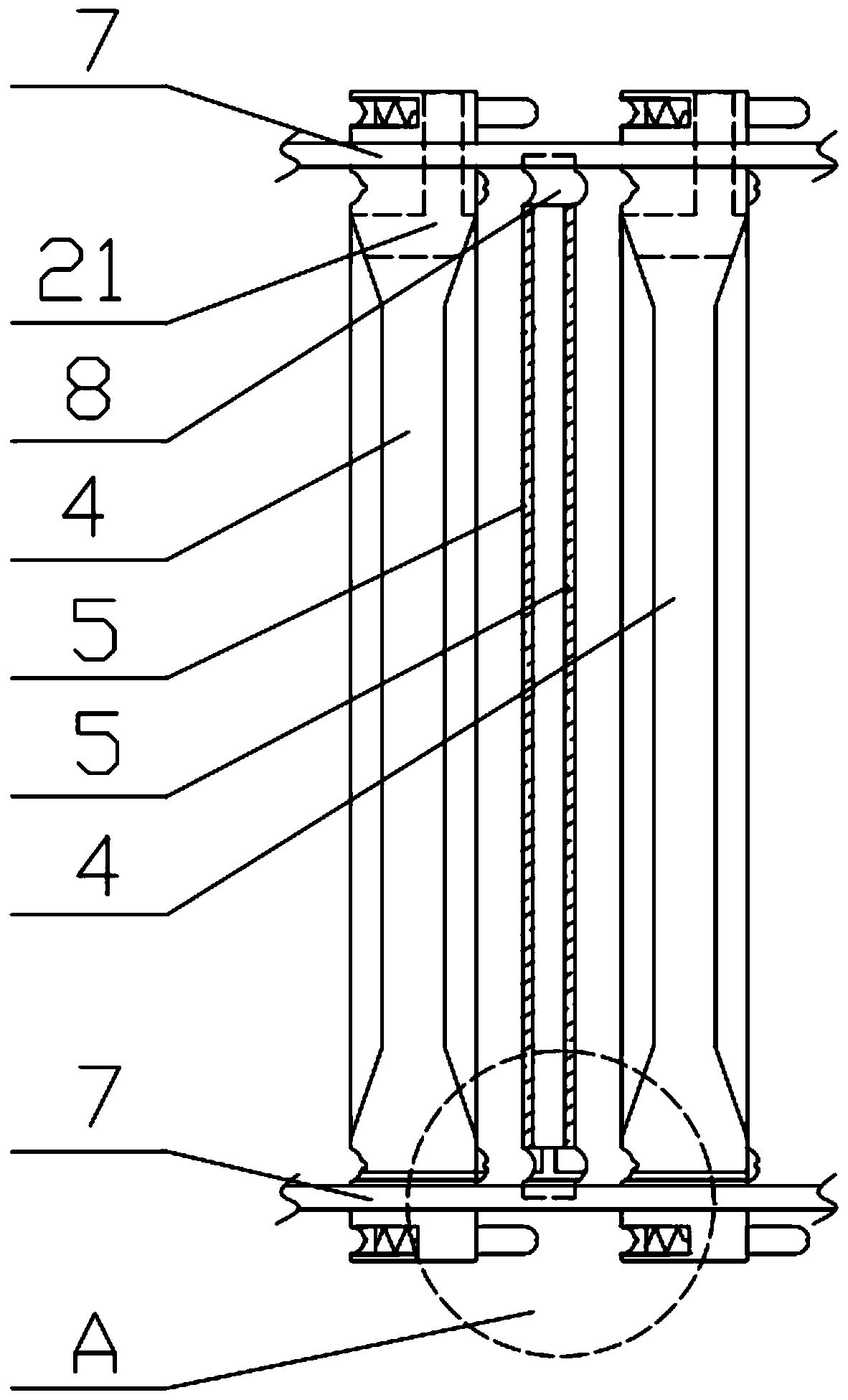
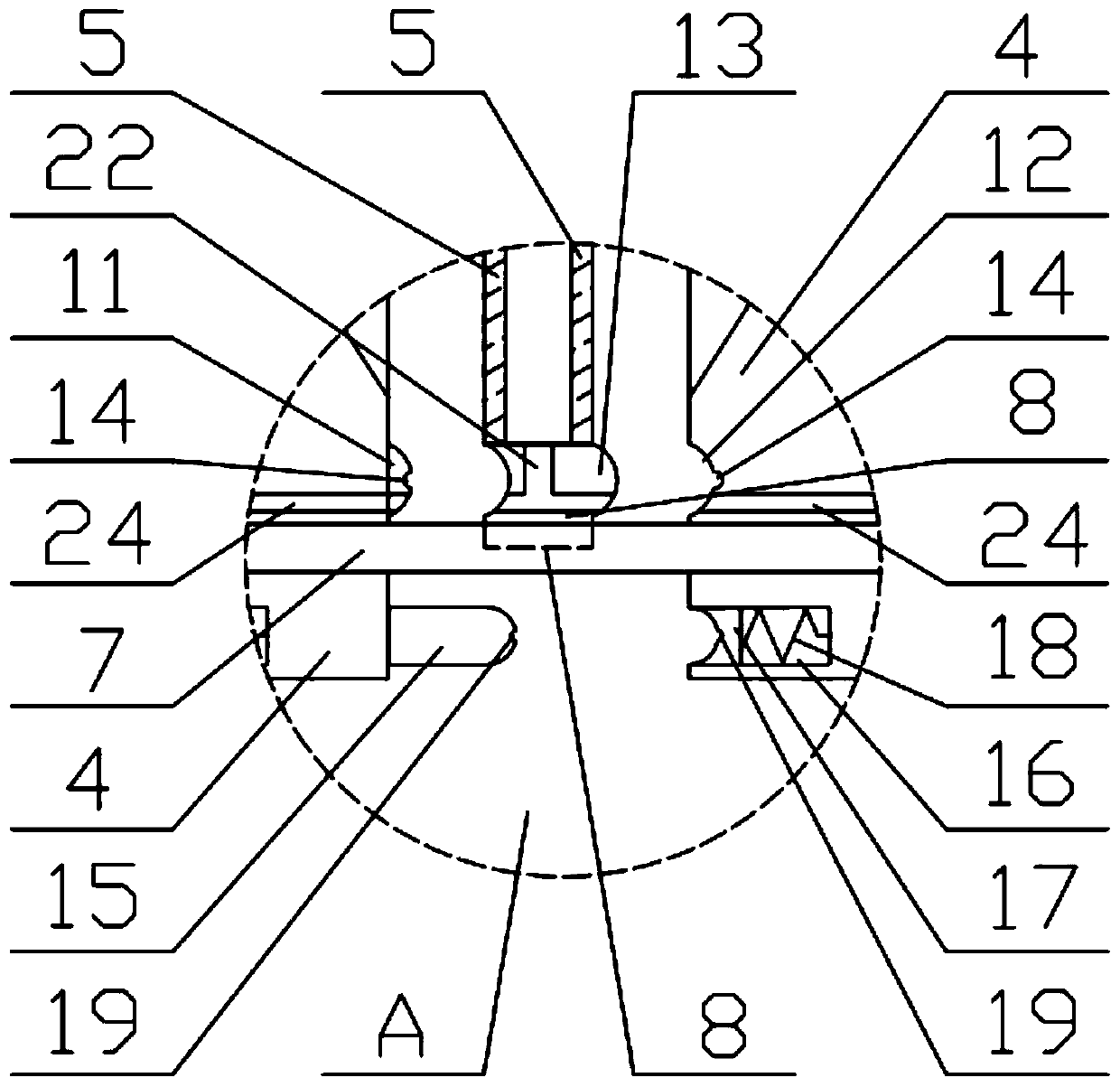
Preparation method of ganciclovir powder injection and filter press used by same
A technology of clovir powder injection and ganciclovir, which is applied in the field of drug production, can solve the problems of inconvenient application, cumbersome operation, and inconvenient disassembly of filter cloth, and achieve the effects of easy collection, simple process, and easy popularization and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] A preparation method of ganciclovir powder injection, is characterized in that, comprises the steps:
[0044] (1) primary refining: adding sodium hydroxide in the crude product of ganciclovir, the mass ratio of the crude product of ganciclovir and sodium hydroxide is 5:1, then adding ethanol solution, the crude product of ganciclovir And the quality of sodium hydroxide and the ratio of the quality with the ethanol solution are 1:8, after stirring and dissolving, filter with gac, obtain filtrate 1, and filtrate 1 evaporates and purifies, obtains solution 1;
[0045] (2) Secondary refining: Put solution one and DMF into the autoclave together, the volume ratio of DMF and solution one is 10:1, heat up to 100°C rapidly, stir for 2h, then cool to 60°C, use Press the filter press to filter, collect the filtrate 2, continue to cool the filtrate 2 to 0° C. and keep it warm for 5 hours, then centrifuge to obtain the high-quality ganciclovir;
[0046] (3) Preparation of medicina...
Embodiment 2
[0058] A preparation method of ganciclovir powder injection, is characterized in that, comprises the steps:
[0059] (1) primary refining: adding sodium hydroxide in the crude product of ganciclovir, the mass ratio of the crude product of ganciclovir and sodium hydroxide is 8:1, then adding ethanol solution, the crude product of ganciclovir And the quality of sodium hydroxide and the ratio of the quality with ethanol solution are 1:10, after stirring and dissolving, filter with gac, obtain filtrate 1, filtrate 1 evaporates and purifies, obtains solution 1;
[0060] (2) Secondary refining: put solution 1 and DMSO into the autoclave together, the volume ratio of DMSO and solution 1 is 10:1, rapidly heat up to 100°C, stir for 2h, then cool to 60°C, use Press the filter press to filter, collect the filtrate 2, continue to cool the filtrate 2 to 0° C. and keep it warm for 5 hours, then centrifuge to obtain the high-quality ganciclovir;
[0061](3) Preparation of medicinal liquid: ...
Embodiment 3
[0065] A preparation method of ganciclovir powder injection, is characterized in that, comprises the steps:
[0066] (1) primary refining: adding sodium hydroxide in the crude product of ganciclovir, the mass ratio of the crude product of ganciclovir and sodium hydroxide is 8:1, then adding ethanol solution, the crude product of ganciclovir And the quality of sodium hydroxide and the ratio with the quality of ethanol solution are 1:12, after stirring and dissolving, filter with gac, obtain filtrate 1, filtrate 1 evaporates and purifies, obtains solution 1;
[0067] (2) Secondary refining: put solution 1 and the composition of DMF and DMSO into the autoclave, the volume ratio of the composition of DMF and DMSO to solution 1 is 10:1, and rapidly heat up to 100°C, Stir for 2 hours, then cool to 60°C, press filter with a filter press, collect the filtrate 2, continue to cool the filtrate 2 to 0°C and keep it warm for 5 hours, then centrifuge to obtain the high-quality ganciclovir;...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com