Use of protease nexin II\KPI protein mutant
A mutant and fusion protein technology, applied in the field of hemorrhagic disease treatment, can solve the problems of different bleeding severity and improve the bleeding severity of hemophiliac patients, and achieve the effects of promoting blood coagulation, good therapeutic application prospects, and low immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
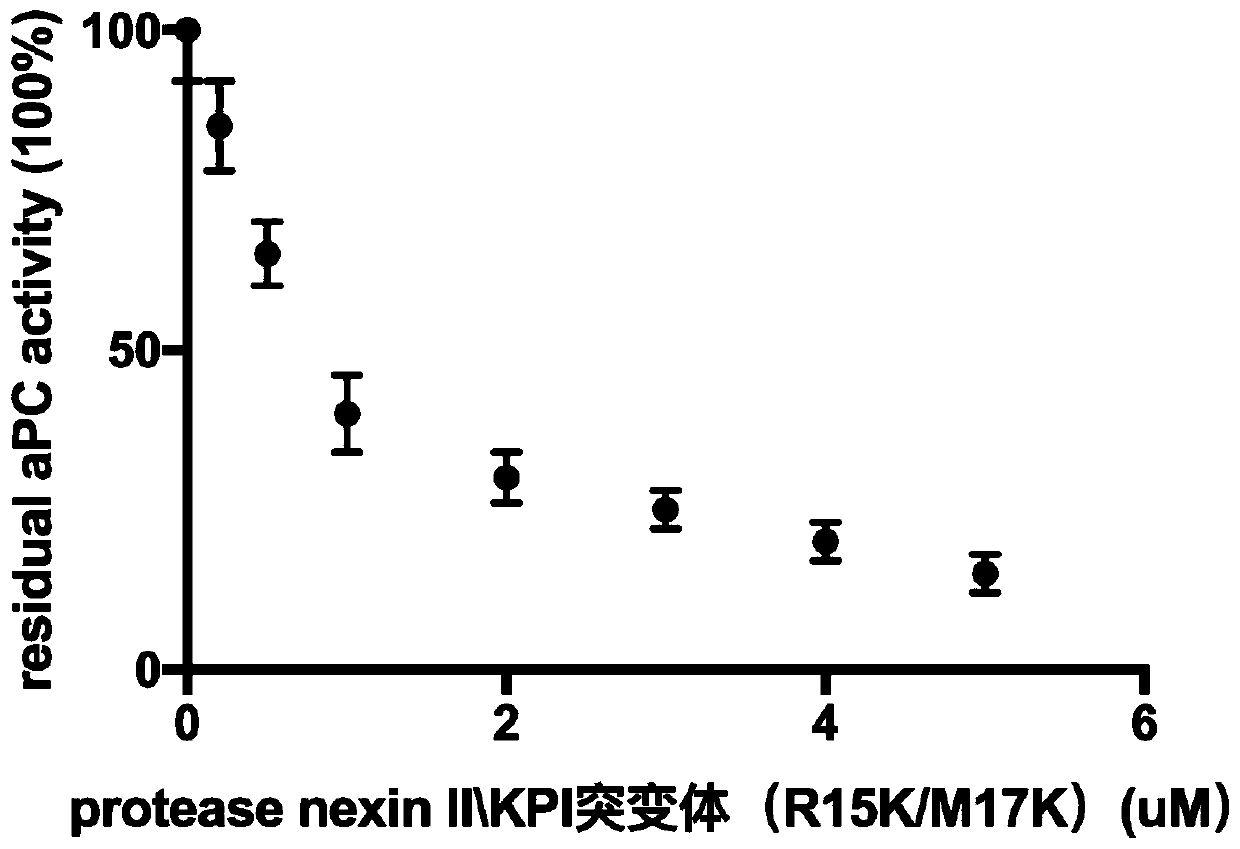
[0025] Inhibition of protein C by protease nexin II\KPI mutant (R15K / M17K)
[0026]In the purified protein system, different concentrations of protease nexin II\KPI mutants (R15K / M17K) were reacted with activated protein C, and the protease nexin II\KPI mutants (R15K / M17K ) inhibited the activity of residual aPC, the results showed that with the increase of the concentration of protease nexin II\KPI mutant (R15K / M17K), the activity of aPC gradually decreased, and the effect of protease nexin II\KPI mutant (R15K / M17K) on aPC has moderate inhibitory activity (IC50~1uM)( figure 1 ).
Embodiment 2
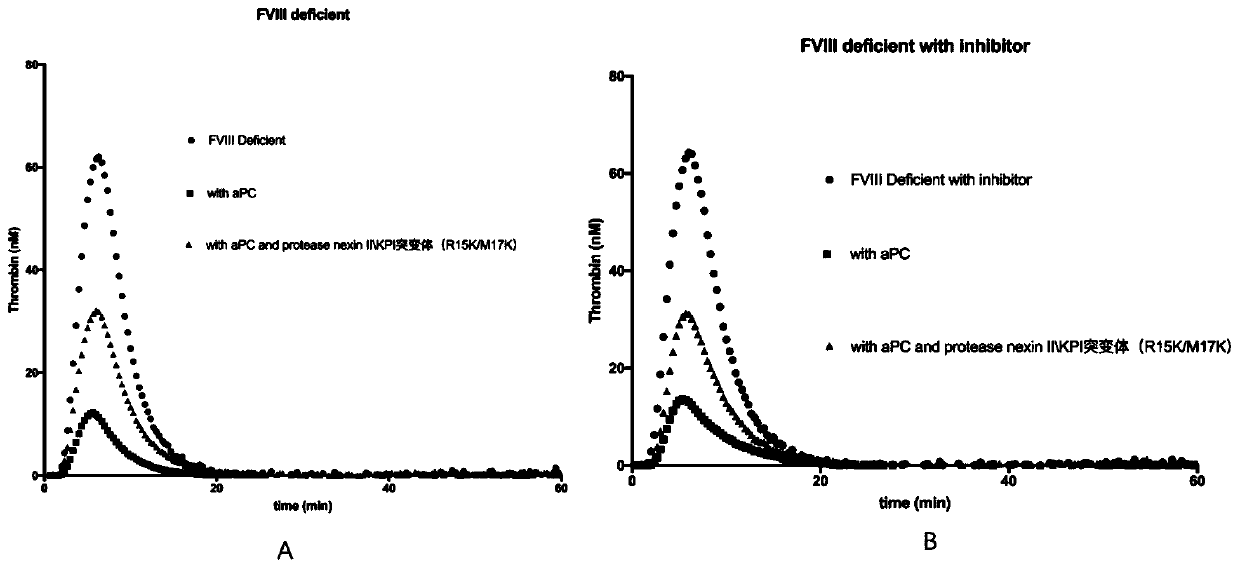
[0028] The protease nexin II\KPI mutant (R15K / M17K) corrects the defect of thrombin generation in the plasma of hemophilia A and B patients and the plasma of hemophilia A patients with inhibitors
[0029] Thrombin generation test (TGT): It can be used to evaluate the thrombin generation ability in plasma. It is an overall coagulation function test that simulates coagulation conditions in vivo, reflecting the total effect of coagulation and anticoagulation systems. Plasma coagulation reaction is started after adding activators (including tissue factor and phospholipids), thrombin generation is detected by specific fluorescent substrate, and the change of fluorescence intensity is dynamically monitored by FLUOROSKAN fluorescence reader, and specific thrombin generation experiment software is used The fluorescent signal is converted into a digital signal, and the thrombin generation curve is drawn. The ability to generate thrombin can be evaluated from several parameters of the g...
Embodiment 3
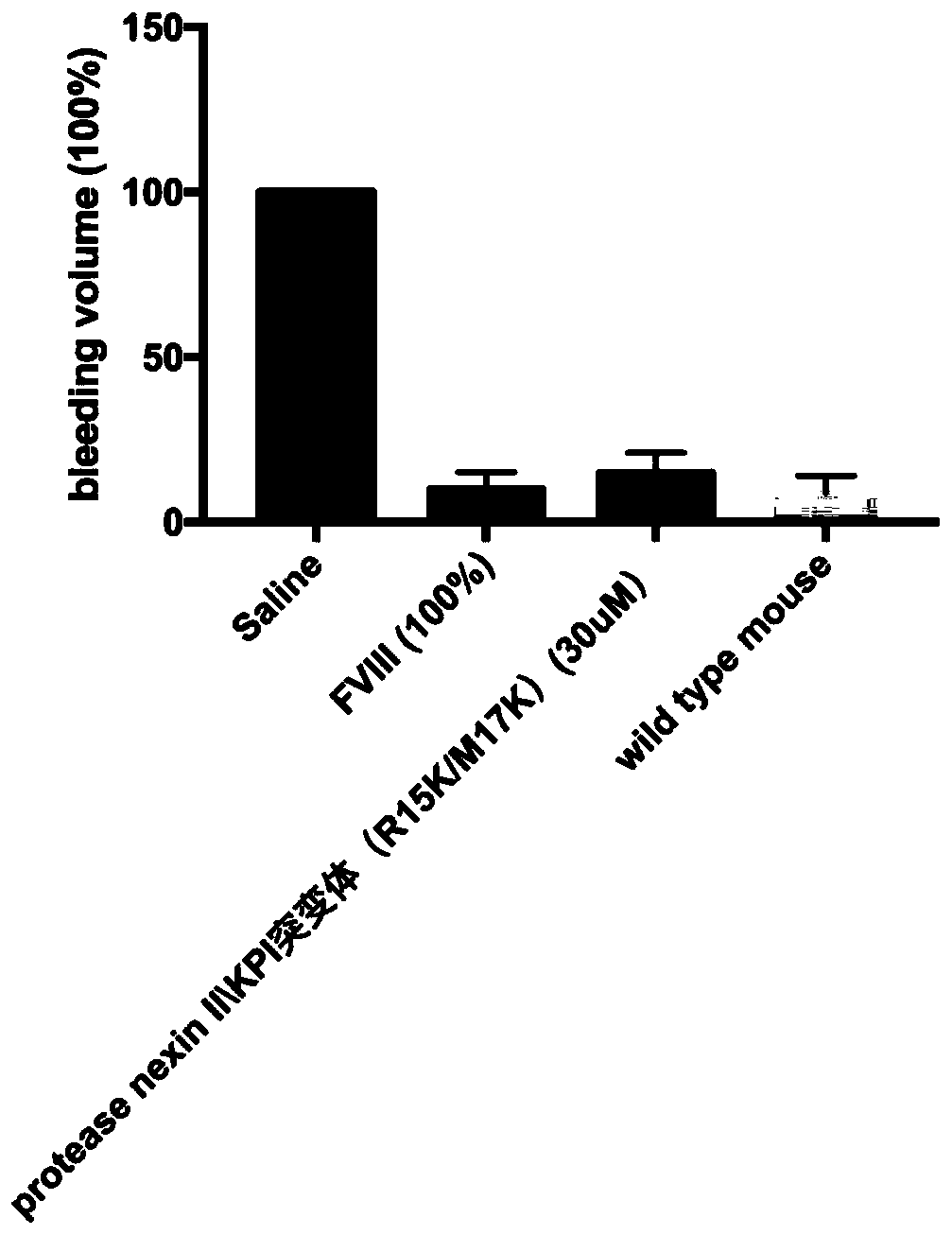
[0032] Protease nexin II\KPI mutant (R15K / M17K) improves tail-docked bleeding phenotype in hemophilic mice
[0033] The protease nexin II\KPI mutant (R15K / M17K) was injected into the mouse model of hemophilia at 4-8 weeks through the tail vein to make the peak concentration of protease nexin II\KPI mutant (R15K / M17K) in plasma up to 30uM. Simultaneous injection of physiological saline was used as a negative control, and blood coagulation factor VIII (peak concentration was 100% of normal) was used as a positive control. After the mouse was anesthetized, the tail was docked at a place with a diameter of 2 mm at the end of the mouse, and the tail was suspended in PBS buffer at room temperature. Timed for 10 minutes, the concentration of hemoglobin in the test tube was detected, and the bleeding volume of the docked tail was calculated according to the hemoglobin measurement. The amount of hemorrhage in saline-treated hemophilic mice was that of the control (100%) ( image 3 )....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com