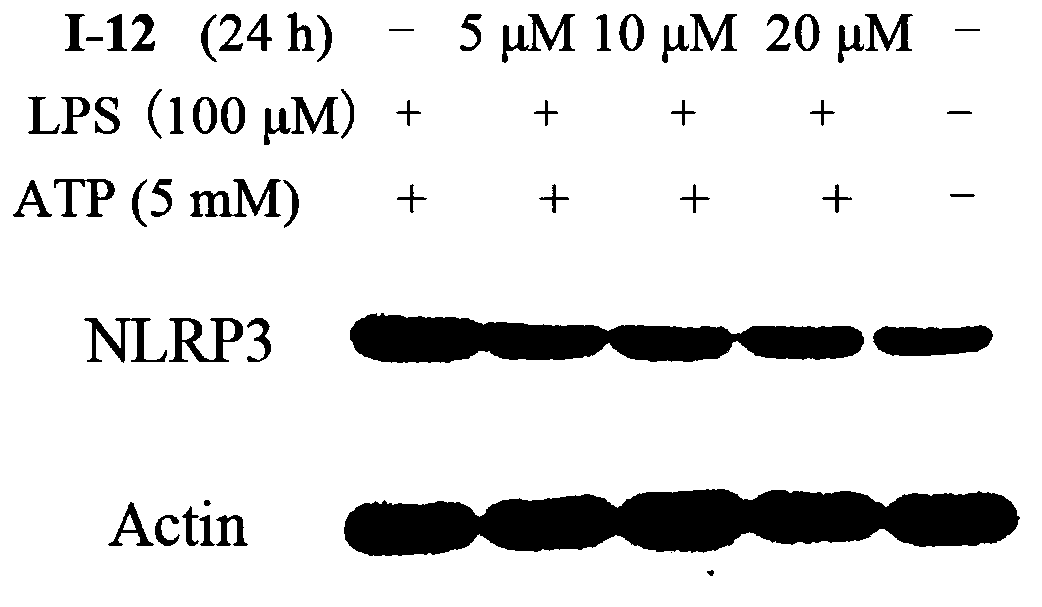
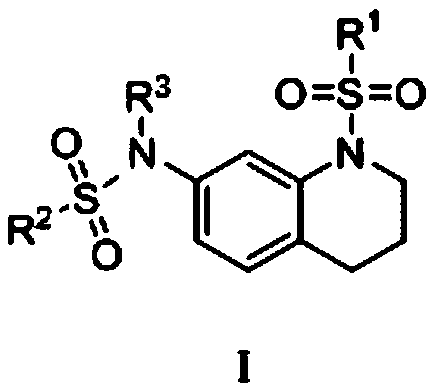
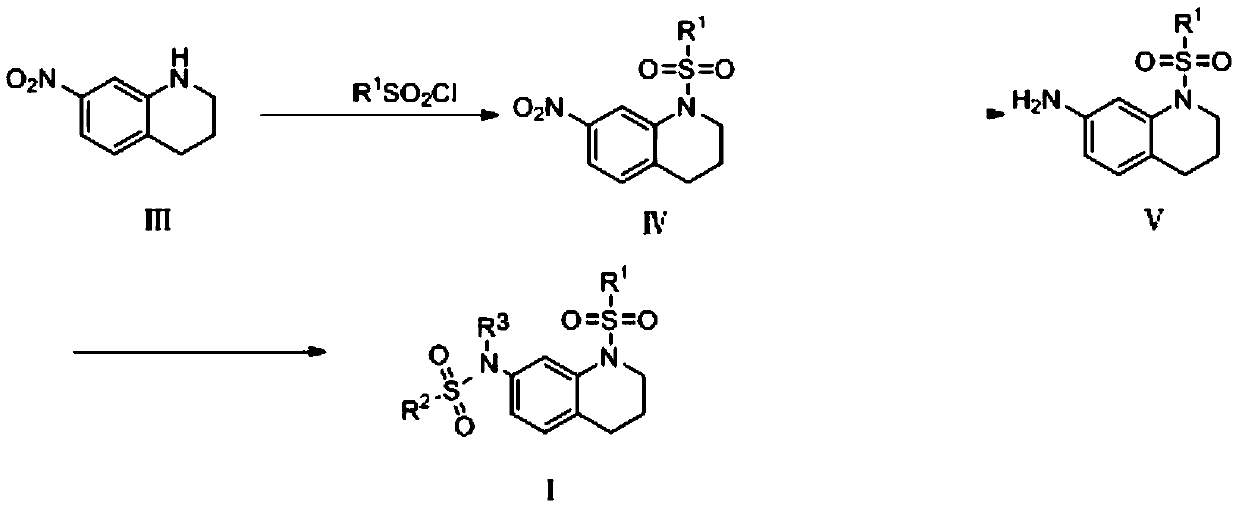
Sulfonamide compound with tetrahydroquinoline as core and preparation method and application of sulfonamide compound
A technology of tetrahydroquinoline and sulfonamide, which can be applied in the directions of active ingredients of heterocyclic compounds, drug combinations, organic chemistry, etc., and can solve problems such as being in the initial stage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment 1 synthetic compound III
[0064]Weigh 7-nitrotetrahydroquinoline (48g, 0.27mol) in a reaction flask, add 100mL of dichloromethane and triethylamine (74.8mL, 0.54mol) to it in turn, stir for 15min under ice bath, dropwise add Methanesulfonyl chloride (21 mL, 0.27 mol), after stirring for 1 h, returned to room temperature and reacted until TLC monitored that the starting material disappeared completely. Wash with dilute hydrochloric acid and saturated brine successively, collect the organic phase, dry over anhydrous sodium sulfate, and remove the solvent under reduced pressure. Add 1g / 1mL of methanol to make slurry for 1h, and filter to obtain 62.9g of white solid, yield: 91.2%. 1 HNMR (300MHz, CDCl 3 )δ8.56(d,J=2.1Hz,1H),7.89(dd,J=8.4,2.2Hz,1H),7.31(d,J=8.2Hz,1H),3.87–3.84(m,2H), 3.03(s,3H),2.97(t,J=6.6Hz,2H),2.17–2.00(m,2H). 13 CNMR (75MHz, CDCl 3 )δ149.34, 140.33, 138.77, 133.03, 121.23, 119.81, 48.84, 41.79, 30.12, 24.34. HRMS (ESI) calcd for C 10 h ...
Embodiment 2
[0065] Embodiment 2 synthetic compound Ⅳ
[0066] Compound III (19 g, 74.2 mmol) was weighed into a reaction flask, and 60 mL of dichloromethane and 1.9 g of 10% palladium on carbon (10% Pd on carbon) were added thereto, and reacted at room temperature for 48 h under a hydrogen atmosphere, and the disappearance of raw materials was monitored by TLC. Filtrate through celite, remove the solvent under reduced pressure, and slurry with methanol (30 mL) to obtain 14 g of white solid with a yield of 83.3%. 1 H NMR (300MHz, CDCl 3 )δ7.11(d,J=2.0Hz,1H),6.90(d,J=8.1Hz,1H),6.45(dd,J=8.1,2.1Hz,1H),3.83–3.75(m,2H), 3.63(s,2H),2.89(s,3H),2.74(t,J=6.6Hz,2H),2.01–1.87(m,2H). 13 C NMR (75MHz, CDCl 3 )δ145.29, 137.48, 130.39, 118.65, 111.98, 108.82, 746.64, 38.35, 26.34, 22.29. HRMS (ESI) calcd for C 10 h 15 N 2 o 2 S[M+H] + 227.0849, found 227.0878. HPLC (10%–100% methanol in water), t R =9.43min, >97.60%.
Embodiment 3
[0067] Embodiment 3 synthetic compound Ⅰ-1
[0068] 4-cyano-N-(1-(methylsulfonyl)-1,2,3,4-tetrahydroquinolin-7-yl)benzenesulfonamide (Ⅰ-1)
[0069] Taking compound Ⅰ-1 as an example, weigh compound Ⅳ (200mg, 0.885mmol) into the reaction flask, add 14mL of dichloromethane and pyridine (71μL, 0.885mmol) in turn, and add 4-cyanobenzenesulfonyl chloride dropwise (178mg, 0.885mmol) in dichloromethane (6mL) was reacted at room temperature overnight, and the disappearance of the starting material was monitored by TLC. The reaction solution was successively washed with dilute hydrochloric acid, saturated sodium bicarbonate solution, and saturated brine, dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure. Slurry with 5 mL of methanol and filter. Obtained off-white solid I-1147mg, yield: 42.5%.
[0070] 4-cyano-N-(1-(methylsulfonyl)-1,2,3,4-tetrahydroquinolin-7-yl)benzenesulfonamide (Ⅰ-1). Off-white solid, yield 42.5% , m.p.172.8-173.9°C. 1 H NM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com