A kind of medicine for treating mammary gland hyperplasia containing flat lying chrysanthemum notoginseng and its preparation method and application
A technology for supine chrysanthemum notoginseng and mammary gland hyperplasia, which is applied to medical preparations containing active ingredients, pharmaceutical formulas, drug combinations, etc. It can solve problems such as deterioration, repeated disease, and inappropriate long-term use by patients, and maintain tissue shape , low price, and the effect of reducing the number of lobular hyperplasia and the number of obliterated glandular ducts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] The preparation method of embodiment 1 preparation 1, comprises the steps:
[0052] (1) Preparation of Ashitaba extract
[0053] Get 5kg of dry whole plant Ashitaba and pulverize it into powder that has passed through a 80-mesh sieve, put it into an extraction tank, add 25L of 80% (v / v) ethanol aqueous solution to it each time, and reflux extraction at 60°C for 2 times. Each time for 2 hours, the two extracts were combined, concentrated under reduced pressure, and freeze-dried to obtain 876.5 g of Ashitina extract powder, which was set aside.
[0054] (2) Preparation of malt extract
[0055] Take 3kg of dry malt (raw) and crush it into a powder that has passed through an 80-mesh sieve, put it into an extraction tank, add 15L of water to it each time, decoct twice at 60°C for 1.5 hours each time, and combine the two extractions solution, concentrated under reduced pressure, and freeze-dried to obtain 330.0 g of malt extract powder, which was set aside.
[0056] (3) Pr...
Embodiment 2
[0060] The preparation method of embodiment 2 preparation 2, comprises the steps:
[0061] (1) Preparation of Ashitaba extract
[0062] Get 5kg of dry whole plant Ashitaba and pulverize it into powder that has passed through a 80 mesh sieve, put it into an extraction tank, add 50L of 80% (v / v) ethanol aqueous solution to it each time, and reflux extraction at 80°C for 2 times, Each time for 1 hour, the two extracts were combined, concentrated under reduced pressure, and freeze-dried to obtain 1035.9 g of Ashitina extract powder, which was set aside.
[0063] (2) Preparation of malt extract
[0064] Take 3kg of dry malt (raw) and crush it into a powder that has passed through an 80-mesh sieve, put it into an extraction tank, add 30L of water to it each time, decoct twice at 80°C for 1 hour each time, and combine the two extractions solution, concentrated under reduced pressure, and freeze-dried to obtain 381.0 g of malt extract powder, which was set aside.
[0065] (3) Prepa...
Embodiment 3
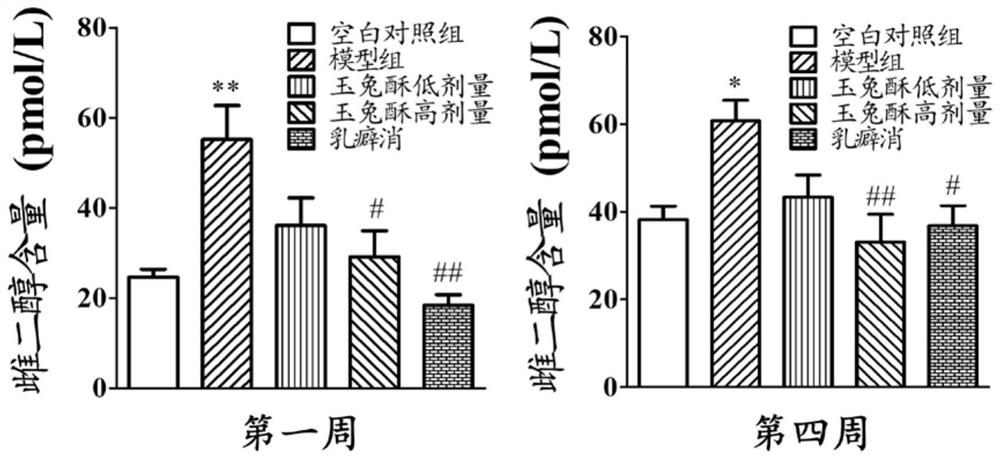
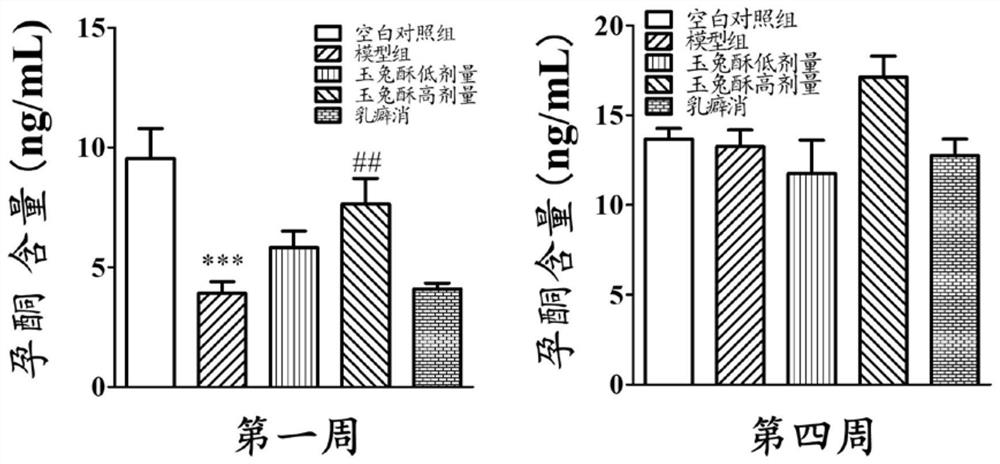
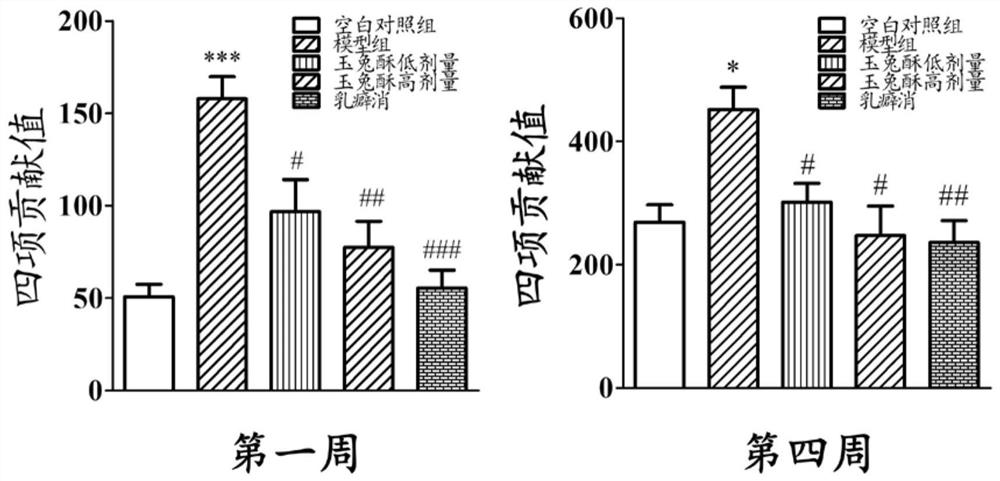
[0068] The therapeutic effect of embodiment 3 preparation 1 on rat mammary gland hyperplasia
[0069] Non-pregnant female SD rats (provided by the Hubei Provincial Center for Disease Control and Prevention), weighing 180 g. Drink water and eat freely, keep the room temperature at 25±1°C, and the light-dark cycle is 12 hours.
[0070] (1) Modeling and administration of mammary gland hyperplasia
[0071] During the experiment period, the pelleted feed was fed, and the experimental period was entered after one week of observational feeding. Female SD rats were randomly divided into 5 groups (5 in each group): blank control group, model group, low-dose group of preparation 1, high-dose group of preparation 1, and Rupixiao group (Rupixiao is a kind of Capsules made of antlers, dandelion, kelp, Panax notoginseng, red peony root, seaweed, leaking reed, woody incense, scrophulariae, motherwort, Caulis Spatholobus, trigeminus, forsythia, gonggongmu, and Smilax tuckahoe, etc., have th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com