Novel method used for determining biological activity of recombinant human growth hormone fusion protein
A technology of biological activity and fusion protein, which is applied in the field of determination of biological activity of recombinant human growth hormone fusion protein, can solve the problems of non-compliance, large coefficient of variation, complicated operation, etc., and achieve small variation, simple operation, and short experiment time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
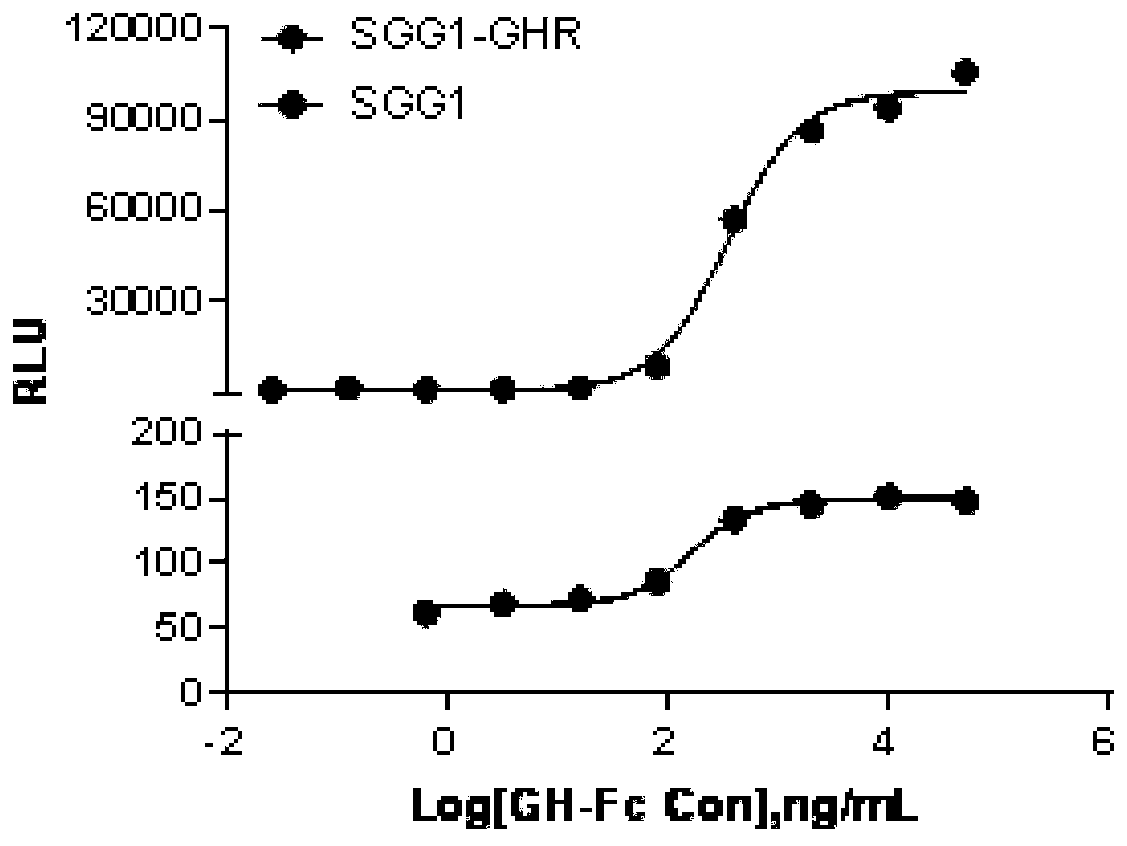
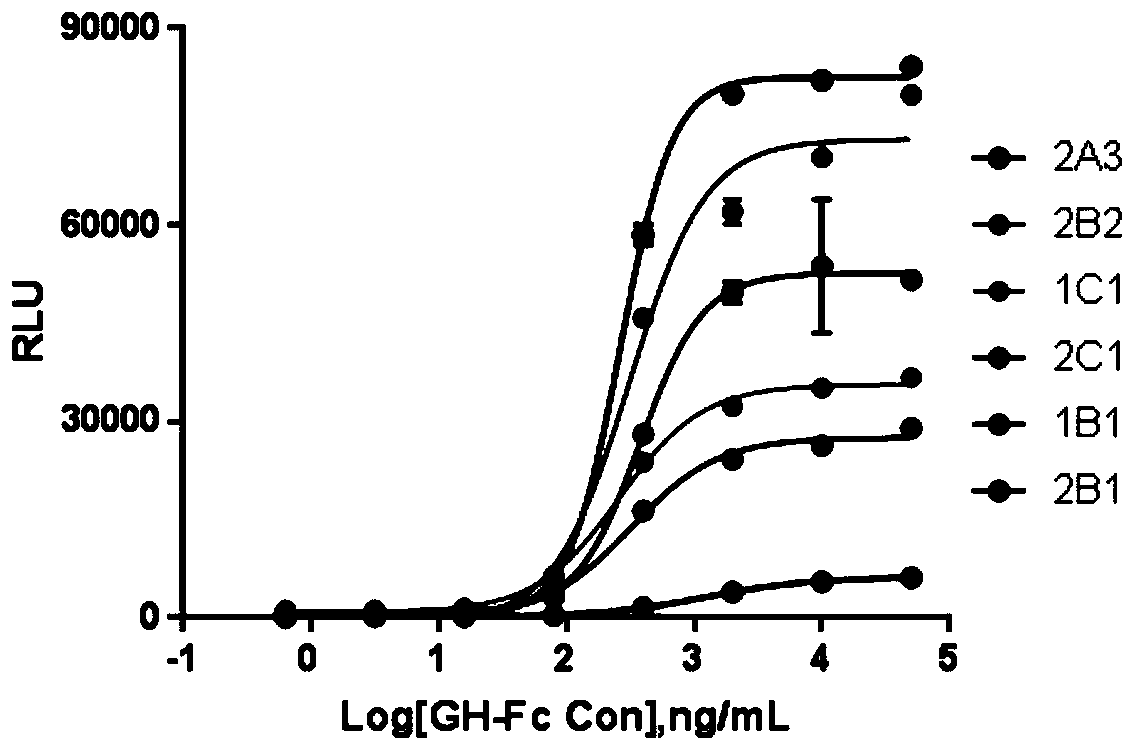
[0034] Example 1 Screening of HEK293 cell lines stably expressing SGG1 and GHR receptors
[0035] 1. Materials and methods
[0036] 1.1 cells
[0037] HEK293 cells were derived from ATCC.
[0038] 1.2 Reagents and materials
[0039] Human growth hormone receptor (GHR) plasmid was purchased from Origene; ViaFect TM Transfection reagents were purchased from Promega; DMEM medium, 1640 medium, fetal bovine serum (FBS) and (G-418) was purchased from GIBCO; hygromycin B was purchased from Suleibao Biotechnology Co., Ltd.; Britelite plus luciferase substrate was purchased from PerkinElmer; white bottom transparent 96-well plate was purchased from CORNING; GH-Fc fusion protein and the plasmid containing the SGG1 response element were retained by the Recombinant Drug Department of the Chinese Academy of Food and Drug Control.
[0040] 1.3 Instrument and analysis software
[0041] A microplate reader was purchased from Molecular devices, model SpectraMax M5; SoftMax Pro and Graph...
Embodiment 2
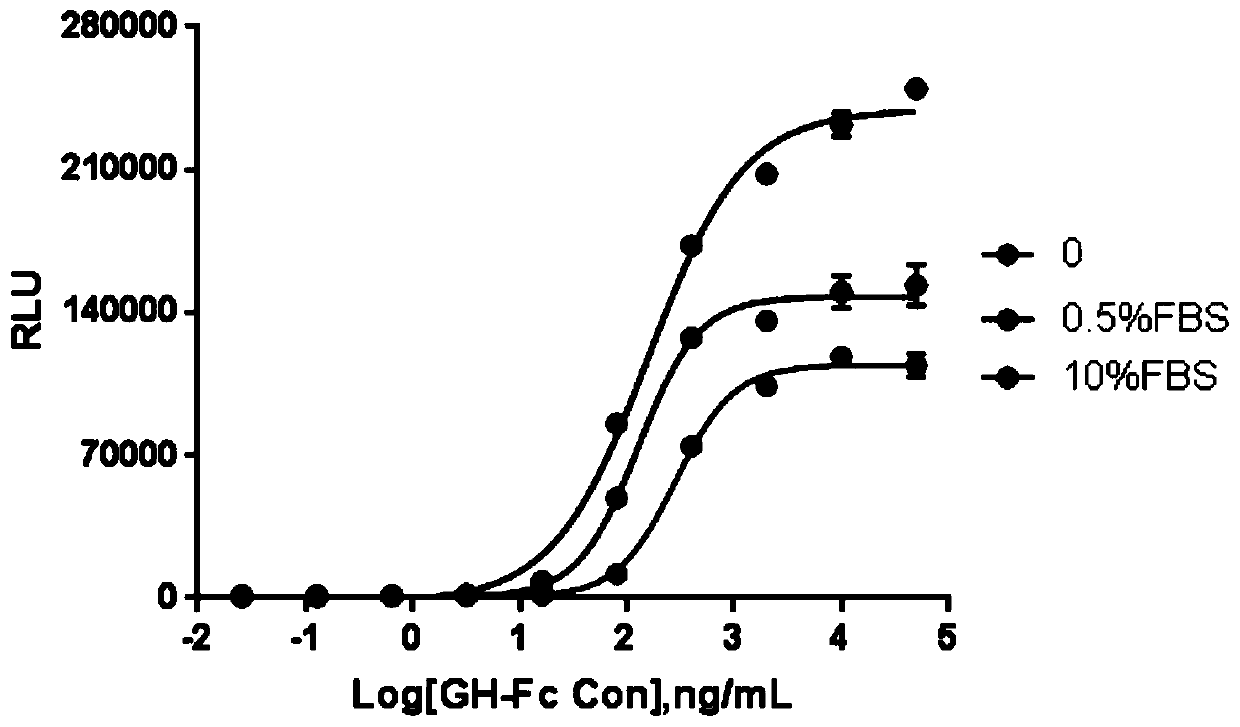
[0053] Example 2 Methodological optimization of GH-Fc fusion protein biological activity assay
[0054] 1. Optimization of the reaction solution Adjust the cell concentration with 1640, 1640 culture medium containing 0.5% FBS and 10% FBS respectively, according to 3×10 4 Add the cells per well into a 96-well plate, culture at 37°C, 5% CO2 for 12-18 hours, add an equal volume of double-diluted GH-Fc fusion protein for stimulation, continue to cultivate for 4-6 hours, discard the reaction solution, and add 60 μL Britelite plus fluorescence Fluorescence value of luciferase was detected after fully shaking for 5 min, and four-parameter curve was fitted ( image 3 ). In view of the principle of minimizing the influence of non-specific substances in the serum on the activity, combined with the SNRs of the three reaction solutions (Table 2), the culture solution containing 1640 was selected as the activity detection reaction solution.
[0055] Table 2 Optimization of reaction solut...
Embodiment 3
[0070] Example 3 Methodological verification of GH-Fc fusion protein biological activity assay
[0071] 1. Exclusiveness
[0072] This method is aimed at the biological activity of the GH-Fc fusion protein. Therefore, different varieties of Fc fusion proteins are used to verify its specificity, including Fc fusion proteins of EPO, VEGFR, IL15 and GLP1. According to the experimental conditions determined in 1-4 in Example 2, the biological activities of the above four Fc fusion proteins were determined.
[0073] see results Figure 7 , this method has no dose-response curves for Fc fusion proteins of EPO, VEGFR, IL15 and GLP1, indicating that this method is not applicable to other drugs except GH-Fc fusion proteins, indicating that the specificity of this method is better.
[0074] 2. Precision
[0075] The precision of the method was evaluated using the experimental conditions determined in Example 2. Four samples of GH-Fc fusion protein were taken for activity determinati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com