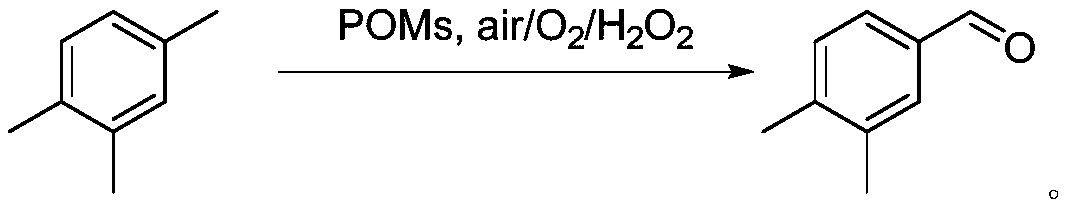Method for preparing 3,4-dimethyl benzaldehyde by efficiently catalyzing pseudocumene
A high-efficiency technology for catalyzing trimethylene and dimethylbenzaldehyde, applied in the field of catalytic chemistry, can solve the problems of no greenness, environmental protection, etc., and achieve the effects of high activity and selectivity, low production cost, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
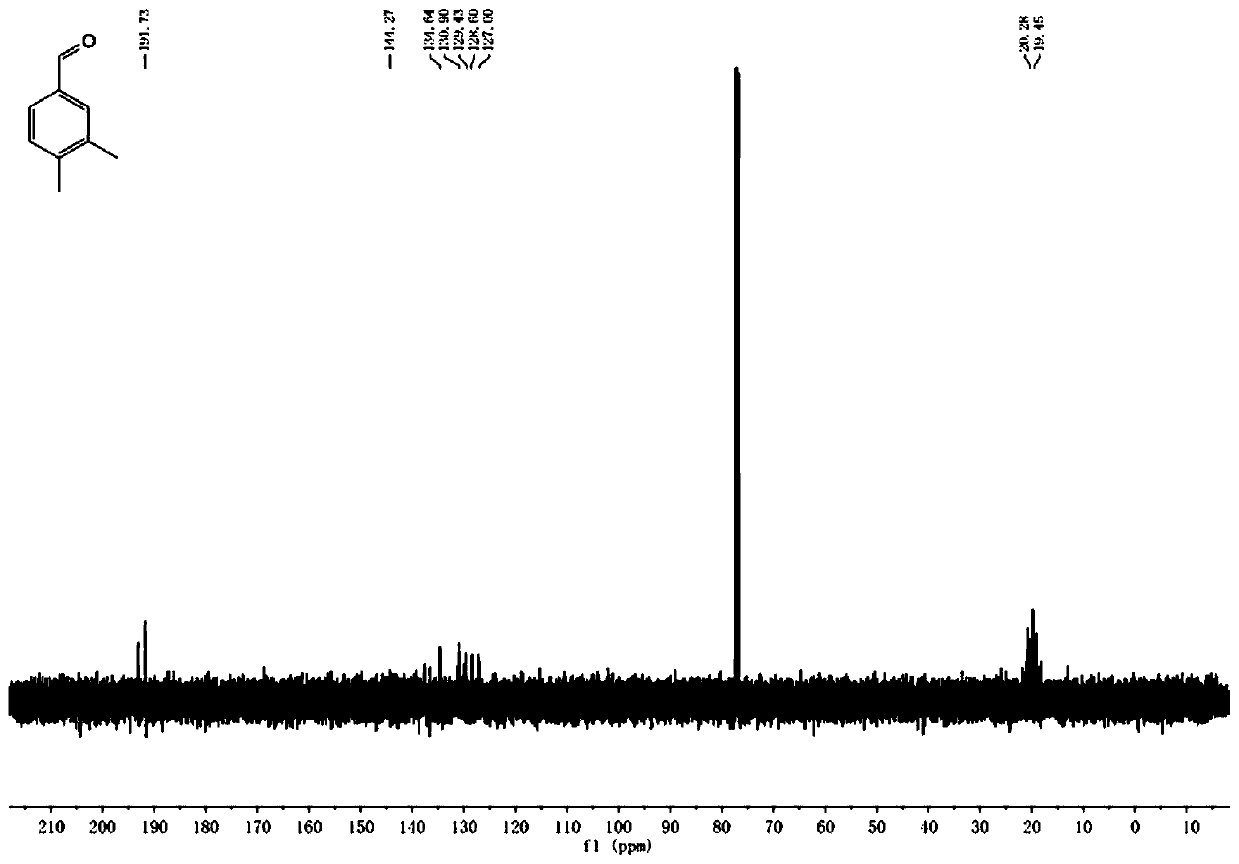
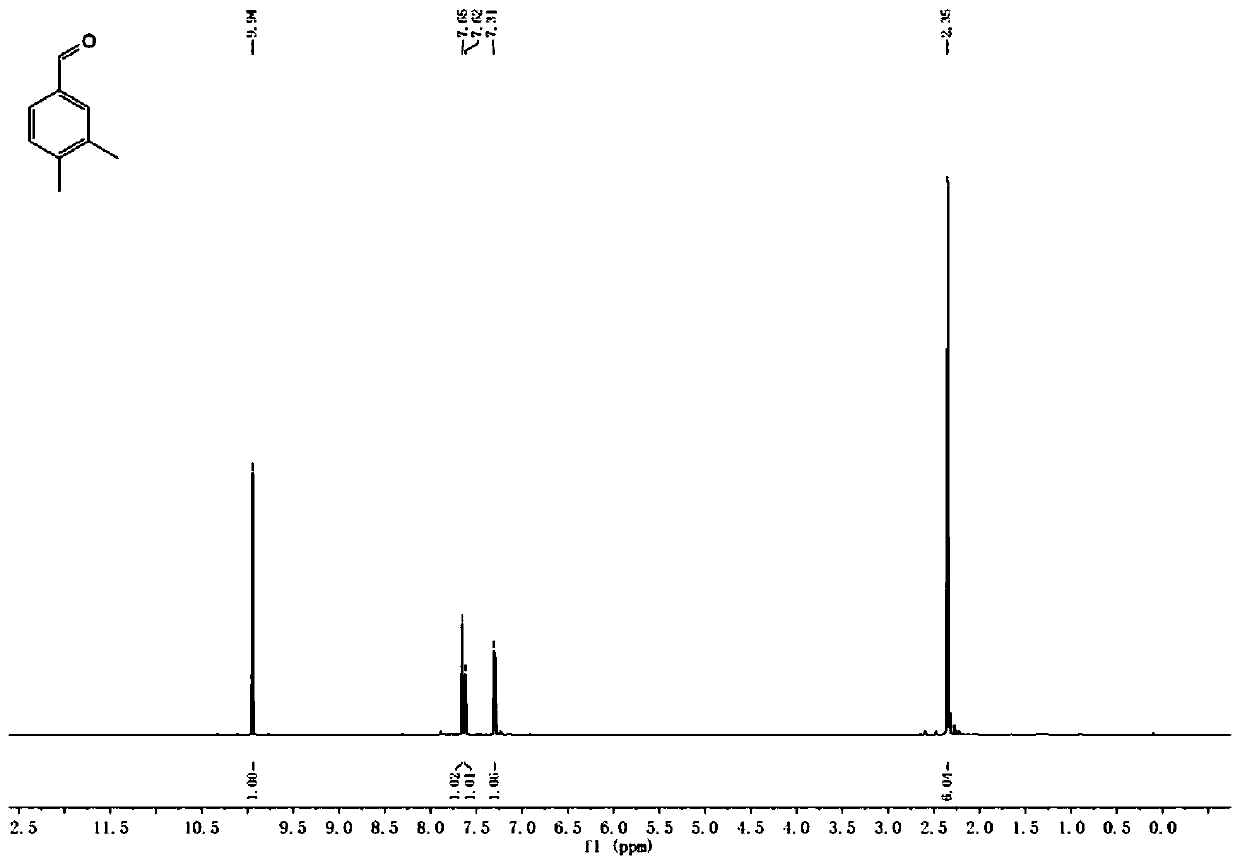
[0024] Put 360g of trimethylbenzene, 0.1~5.0mol% of Fe as the center metal Anderson type polyoxometalate, 2.0~6.0equiv of hydrogen peroxide, and 2.0~6.0L of solvent acetonitrile into the dry reaction tube, and react The tube was covered with a balloon, and the reaction temperature was controlled at 50-80°C. After 24 hours of heat preservation, the reaction was stopped, cooled to room temperature, and the sample was prepared for GC-MS detection. The result of GC-MS was that the conversion rate of the reaction substrate was 95%. After separation and purification, the NMR test was carried out, and it was proved to be 3,4-dimethylbenzaldehyde by the obtained hydrogen spectrum and carbon spectrum data.
Embodiment 2
[0026] 360g of trimethylbenzene, 0.1-5.0mol% of Al as the center metal Anderson type polyoxometalate, 2.0-6.0equiv of hydrogen peroxide, and 2.0-6.0L of solvent acetonitrile are put into the drying reaction tube, and the reaction The tube was covered with a balloon, and the reaction temperature was controlled at 50-80°C. After 24 hours of heat preservation, the reaction was stopped, cooled to room temperature, and the sample was prepared for GC-MS detection. The GC-MS result showed that the conversion rate of the reaction substrate was 93%. After separation and purification, the NMR test was carried out, and it was proved to be 3,4-dimethylbenzaldehyde by the obtained hydrogen spectrum and carbon spectrum data.
Embodiment 3
[0028] 360g of mesitylene, 0.1 to 5.0 mol% of Cr as the Anderson type polyoxometalate of the central metal, 2.0 to 6.0 equiv of hydrogen peroxide, and 2.0 to 6.0 L of solvent acetonitrile are put into a dry reaction tube, and the reaction The tube was covered with a balloon, and the reaction temperature was controlled at 50-80°C. After 24 hours of heat preservation, the reaction was stopped, cooled to room temperature, and the sample was prepared for GC-MS detection. The result of GC-MS was that the conversion rate of the reaction substrate was 97%. After separation and purification, the NMR test was carried out, and it was proved to be 3,4-dimethylbenzaldehyde by the obtained hydrogen spectrum and carbon spectrum data.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com