Method for preparing biomass fuel oil molecules from biomass platform compound through hydrogenation
A technology of biomass fuel oil and compounds, applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, biological raw materials, etc., can solve problems such as catalyst deactivation, and achieve simple preparation process and simple reaction process , highly selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The preparation of embodiment 1 catalyst
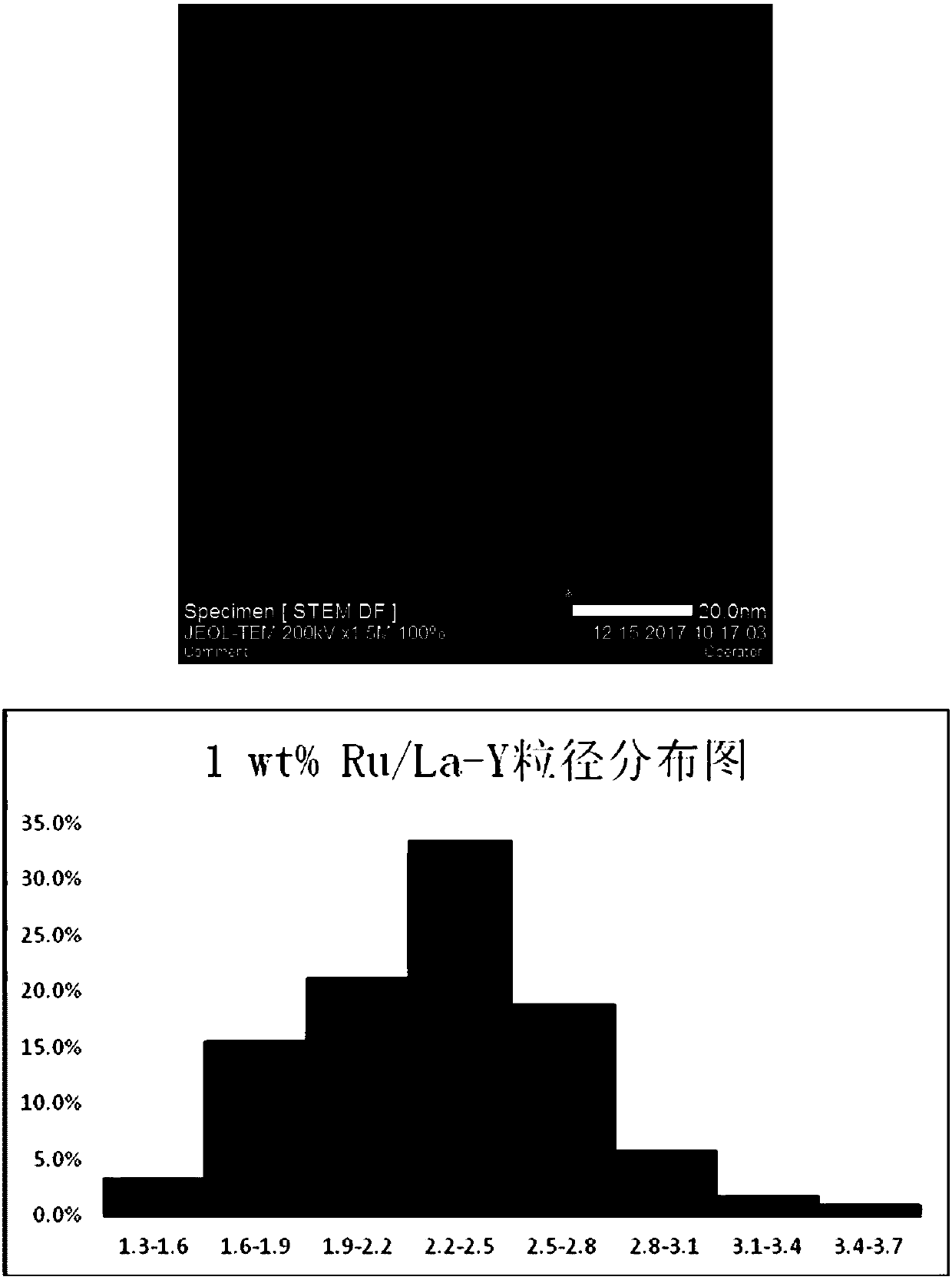
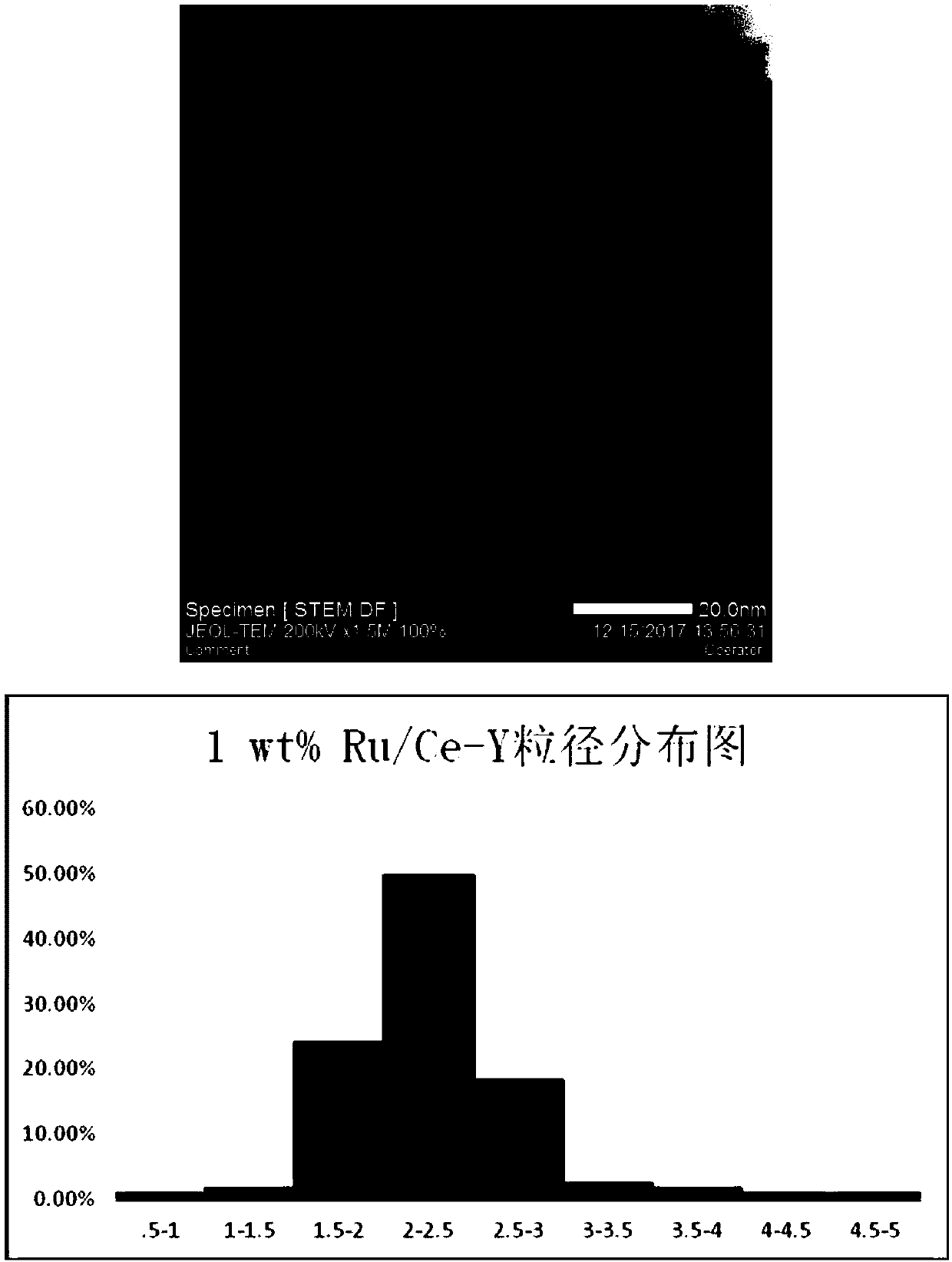
[0023] Experiments 1 and 2: Preparation of La-Y and Ce-Y modified carriers: add 0.8476g of LaCl to 100ml round bottom flasks respectively 3 , 0.8517g of CeCl 3 , add 30ml of ultrapure water to dissolve, then add 2.1524g and 2.1482g of Na-Y molecular sieve respectively, stir the obtained mixed solution in a water bath at 50°C for 2 hours, then stir overnight at 80°C until the water evaporates to dryness, and then Calcined at 600° C. for 2 hours to obtain 3 g of 16 wt % La-Y, 16 wt % Ce-Y carrier modified with rare earth elements.
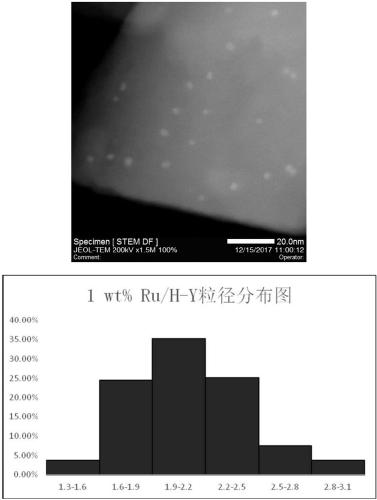
[0024] Experiments 3 and 4: Preparation of H-Y and H-ZSM-5 modified carriers: Weigh 3g of Na-Y and ZSM-5 molecular sieves into 100ml round bottom flasks respectively, and then add 0.10mol / L NH 4 Cl solution 25ml, the resulting mixed solution was kept in a water bath at 50°C for 1 hour, filtered, washed, and then washed with 0.10mol / L NH 4 The Cl solution was ion-exchanged five times, filtered, wash...
Embodiment 2
[0030] Embodiment 2 levulinic acid ester hydrogenation prepares gamma-valerolactone reaction
[0031] Experiment 16-19: Add 5g of ethyl levulinate, or 5g of levulinic acid ester, 45g of solvent, and 0.5g of catalyst into a 50ml reaction kettle, replace the gas with nitrogen for three times, then replace with hydrogen for three times, and then fill Hydrogen to 6MPa, heated to 200°C and stirred for 6 hours. After the reaction was completed, it was lowered to room temperature, and the supernatant liquid was taken and filtered, and the product was detected on an Agilent 7890B gas chromatograph. Calculate the yield of valeric acid, ethyl valerate, gamma-valerolactone, levulinic acid, etc. in the product, and the experimental results are shown in Table 1 (Experiments 16-19).
[0032] The experimental results are shown in Table 1 (Experiments 16-19).
[0033] Table 1 Experimental results of selective hydrogenation of ethyl levulinate to γ-valerolactone with different catalysts.
...
Embodiment 3
[0036] Example 3 Preparation of valeric acid esters by selective hydrogenation of gamma-valerolactone
[0037] The preparation process of 1wt% Ru / La-Y, 1wt% Ru / Ce-Y, and 1wt% Ru / H-Y catalyst is the same as that described in Example 1.
[0038] Experiment 20-22: Add 5g of γ-valerolactone and 0.5g of catalyst into a 50ml reactor, pass through nitrogen to replace the gas three times, then pass through hydrogen to replace three times, then fill with hydrogen to 4MPa, heat up to 220°C and stir for 10 hours . After the reaction was completed, it was lowered to room temperature, and the supernatant liquid was taken and filtered, and the product was detected by Agilent 7890B gas chromatography. The yields of valeric acid, ethyl valerate, gamma-valerolactone, and levulinic acid in the product were calculated, and the experimental results were shown in Table 2 (Experiment 20-22).
[0039] Table 2 Experimental results of preparing valerate by hydrogenation of γ-valerolactone over metal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com