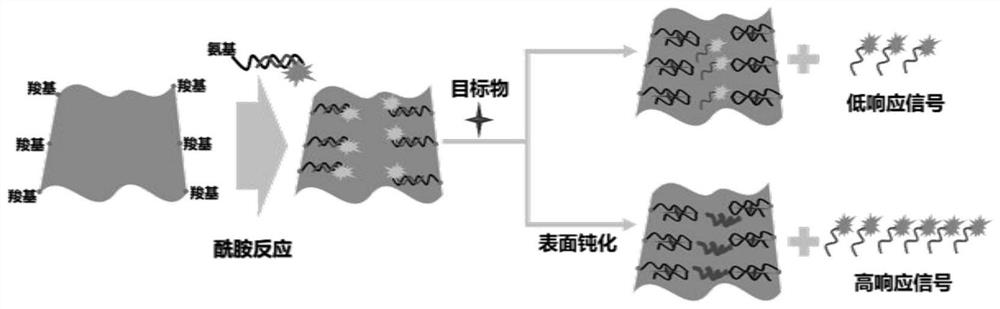
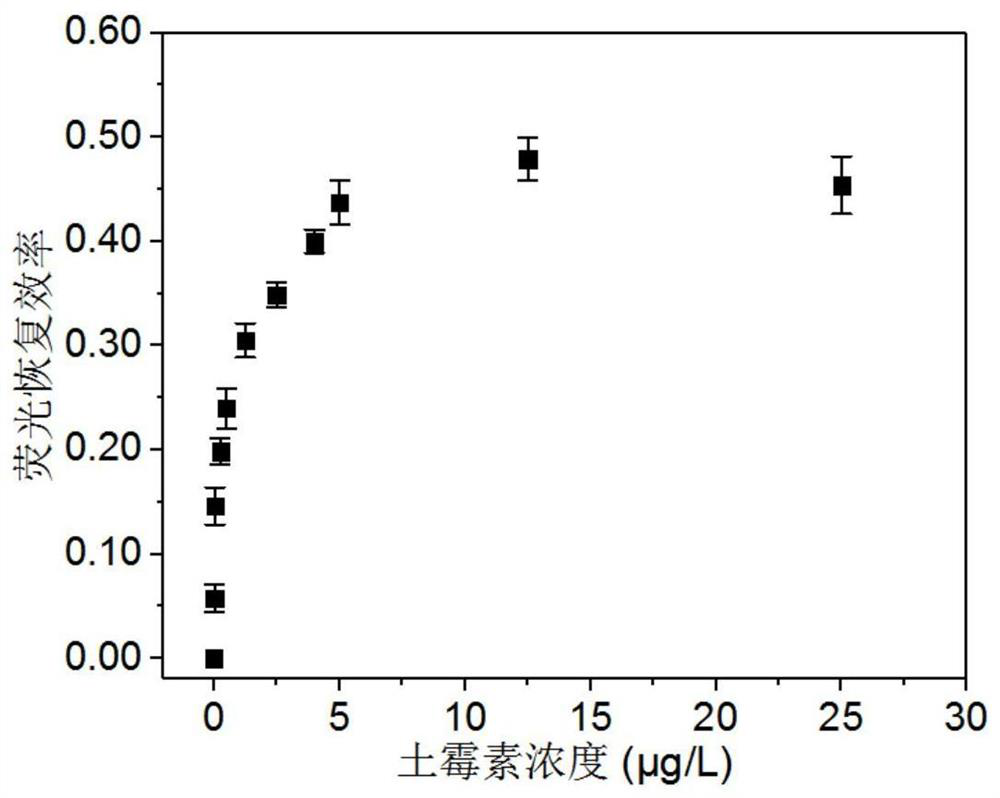
A Fluorescence Detection Method of Oxytetracycline Based on Surface Passivation and Covalent Coupling
A technology of covalent coupling and fluorescence detection, which is applied in the fields of fluorescence/phosphorescence, measurement devices, and material analysis through optical means, and can solve problems such as poor detection performance, low fluorescence recovery efficiency, and high background signal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Configure the determination of oxytetracycline content in the water sample:
[0029] (1) Preparation of metal-organic framework nanosheets (Cu(HBTC)-1):
[0030] Add copper chloride (CuCl 2 ) (2.5M) was added 0.1 g of polyvinylpyrrolidone (PVP, K30). After the mixture was stirred for 5min, 2.5mL of sodium hydroxide (0.2M) was slowly added dropwise, and after stirring for 10min, 2.5mL of freshly prepared ascorbic acid (0.1M) was slowly added dropwise, and the stirring was continued for 10min. Cubic cuprous oxide (Cu 2 O) Nanoparticles. Subsequently, the solid was collected by centrifugation under the condition of 8000 r / min, washed with ethanol for 3 times, and then dispersed into 10 mL of ethanol solution again. Then weigh 0.4g PVP and dissolve it in 60mL high-purity water, and add 4mL trimesic acid ethanol solution (0.24M). After the mixture was stirred for 10 min, the Cu prepared above was added 2 O ethanol dispersion, the mixture turned into a transparent color...
Embodiment 2
[0042] Determination of oxytetracycline content in tap water samples:
[0043] (1) Preparation of metal-organic framework nanosheets (Cu(HBTC)-1):
[0044] Add copper chloride (CuCl 2 ) (2.5M) was added 0.1 g of polyvinylpyrrolidone (PVP, K30). After the mixture was stirred for 5min, 2.5mL of sodium hydroxide (0.2M) was slowly added dropwise, and after stirring for 10min, 2.5mL of freshly prepared ascorbic acid (0.1M) was slowly added dropwise, and the stirring was continued for 10min. Cubic cuprous oxide (Cu 2 O) Nanoparticles. Subsequently, the solid was collected by centrifugation under the condition of 8000 r / min, washed with ethanol for 3 times, and then dispersed into 10 mL of ethanol solution again. Then weigh 0.4g PVP and dissolve it in 60mL high-purity water, and add 4mL trimesic acid ethanol solution (0.24M). After the mixture was stirred for 10 min, the Cu prepared above was added 2 O ethanol dispersion, the mixture turned into a transparent color within 2min....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com