Cabazitaxel protein nanomaterial and preparation method thereof
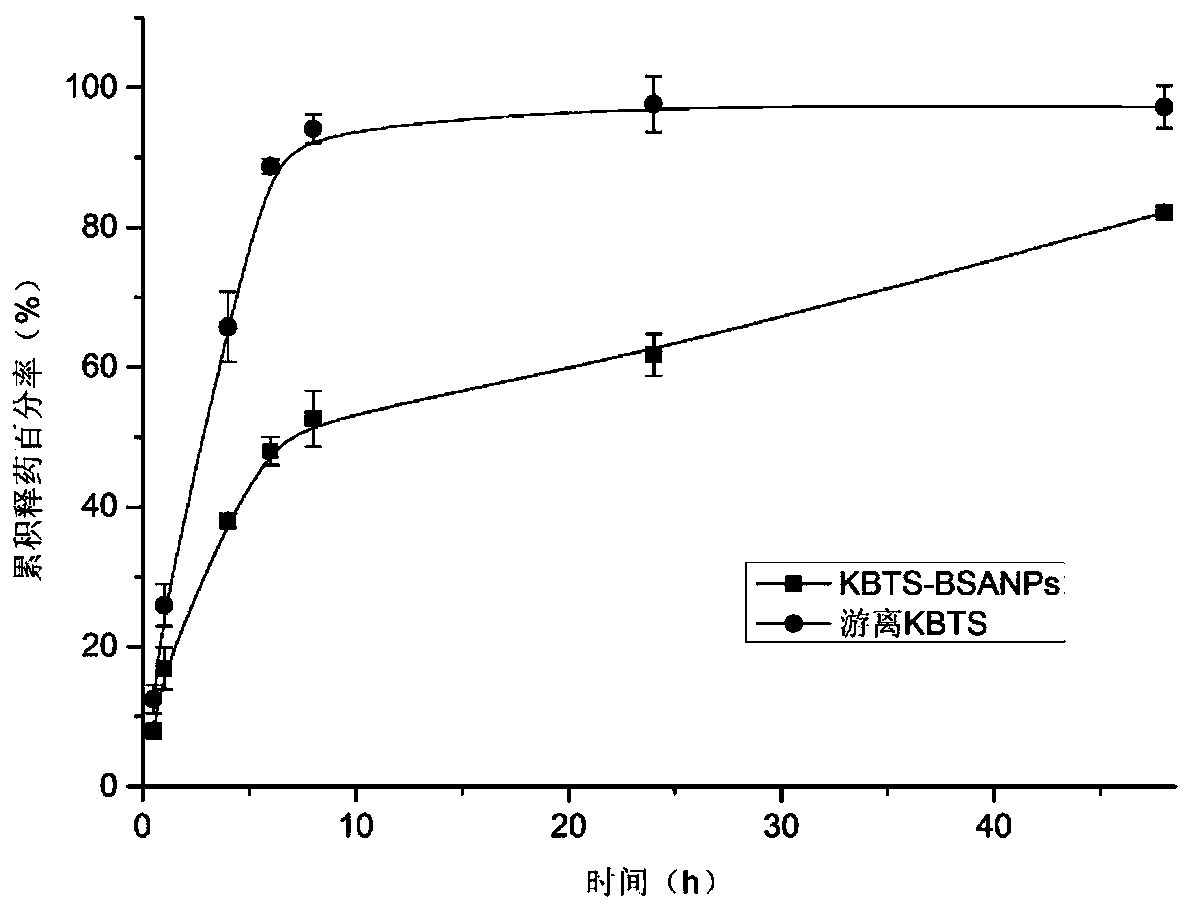
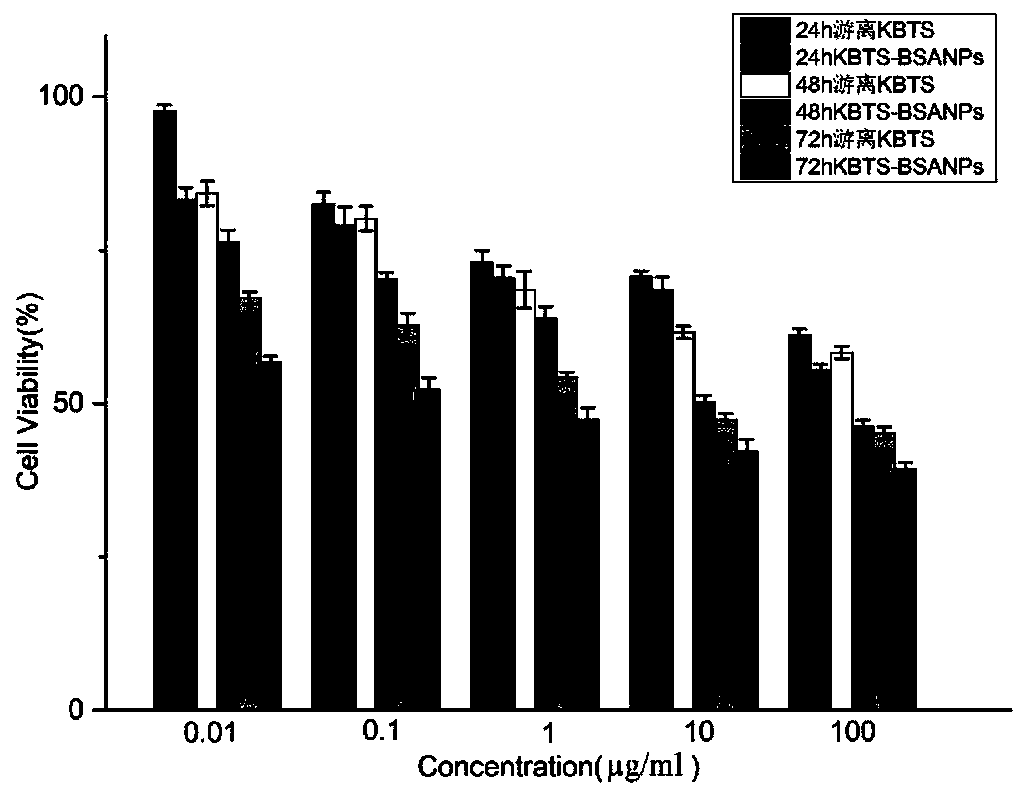
A technology of cabazitaxel and nanomaterials, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve toxic and side effects, hypersensitivity reactions, hidden dangers of drug safety, and dosage Large and other problems, to achieve the effect of prolonging the administration time, reducing the administration dose, and high encapsulation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Cabazitaxel protein nanomaterials, including the following raw materials:
[0035] Cabazitaxel 50mg; Cholesterol 100mg; Bovine serum albumin 0.5g (0.5% concentration).
[0036] Preparation Process:
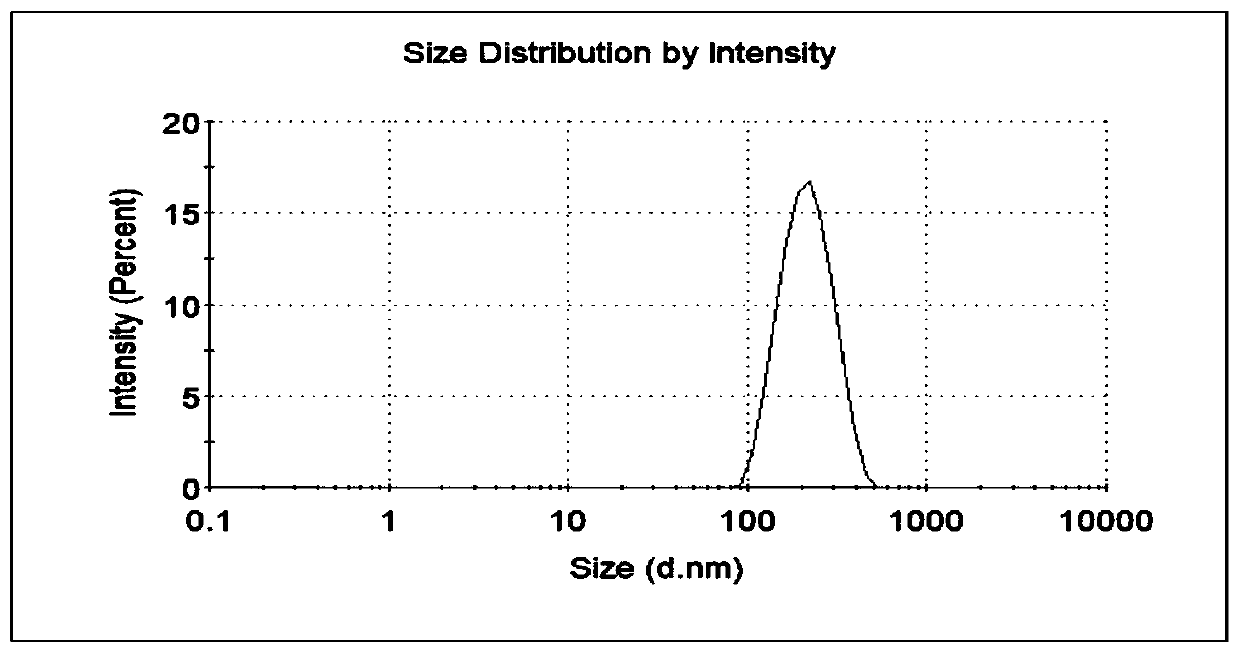
[0037] Dissolve cabazitaxel and cholesterol in an organic solvent (chloroform: absolute ethanol = 11:1, 10ml in total), and use it as an oil phase for later use; dissolve bovine serum albumin in ultrapure water, adjust the pH to 8, Standby as the water phase (oil-water ratio 1:10); Add the oil phase into the water phase, and perform ultrasonication in an ice bath at 40% power for 8 min to form a primary emulsion; pass the primary emulsion through a high-pressure homogenizer at a pressure of Under the condition of 500bar, cycle 8 times; the treated emulsion was subjected to vacuum rotary evaporation at 35°C to remove the organic solvent, and centrifuged at 22000r / min (34700×g) for 30min at 4°C to remove the supernatant and precipitate Add proper amount of ultrapure water t...
Embodiment 2
[0039] Cabazitaxel protein nanomaterials, including the following raw materials:
[0040] Cabazitaxel 100mg; cholesterol 150mg; bovine serum albumin 1.5g (1.5%).
[0041] Preparation Process:
[0042] Dissolve cabazitaxel and cholesterol in an organic solvent (chloroform: absolute ethanol = 11:1, 10ml in total), and use it as an oil phase for later use; dissolve bovine serum albumin in ultrapure water, adjust the pH to 10, Standby as the water phase; add the oil phase to the water phase, and perform ultrasonication in an ice bath at 30% power for 12 minutes to form a primary emulsion; pass the primary emulsion through a high-pressure homogenizer at a pressure of 800 bar, and circulate it 8 times The treated emulsion is subjected to 35°C decompression rotary steaming process to remove the organic solvent, centrifuged at 22000r / min (34700×g) for 30min at 4°C, removes the supernatant, and adds an appropriate amount of ultrapure water to the precipitate for redissolution. have t...
Embodiment 3
[0044] Cabazitaxel protein nanomaterials, including the following raw materials:
[0045]Cabazitaxel 150mg; cholesterol 200mg; bovine serum albumin 1.5g (1.5%).
[0046] Preparation Process:
[0047] Dissolve cabazitaxel and cholesterol in an organic solvent (chloroform: absolute ethanol = 11:1, 10ml in total), and use it as an oil phase for later use; dissolve bovine serum albumin in ultrapure water, adjust the pH to 8, Standby as the water phase; add the oil phase into the water phase, and perform ultrasonication in an ice bath at 40% power for 8 minutes to form a primary emulsion; pass the primary emulsion through a high-pressure homogenizer under the condition of a pressure of 500 bar, and circulate 8 times ; The processed emulsion was subjected to vacuum rotary evaporation at 35°C to remove the organic solvent, centrifuged at 22,000 r / min (34,700×g) and 4°C for 30 minutes, removed the supernatant, and reconstituted the precipitate with an appropriate amount of ultrapure ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com