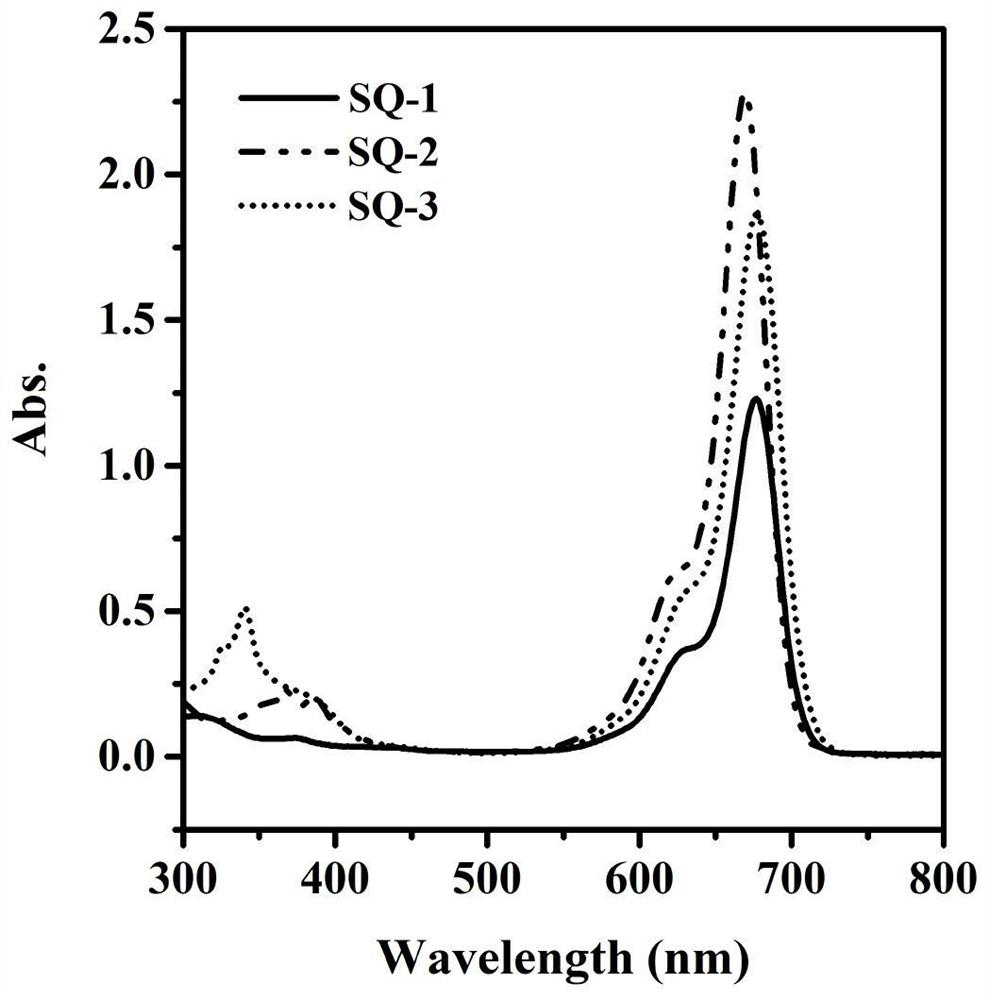
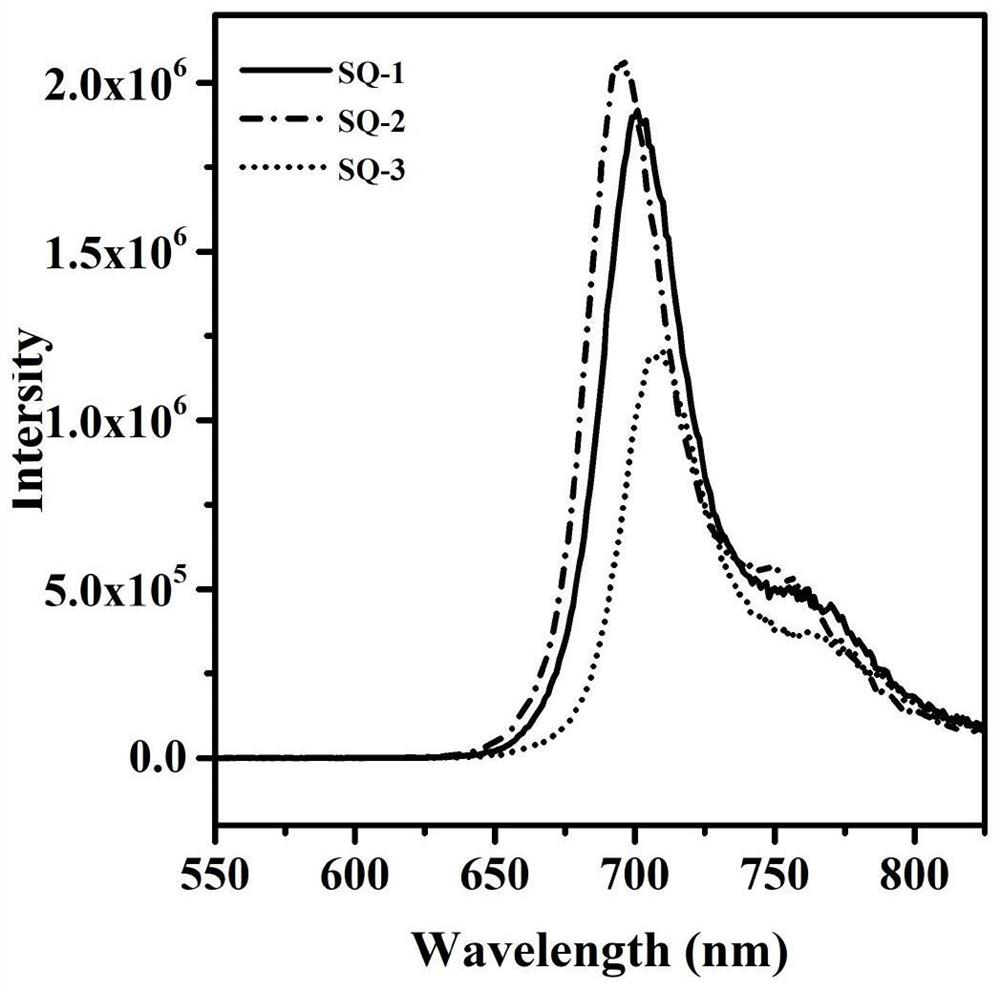
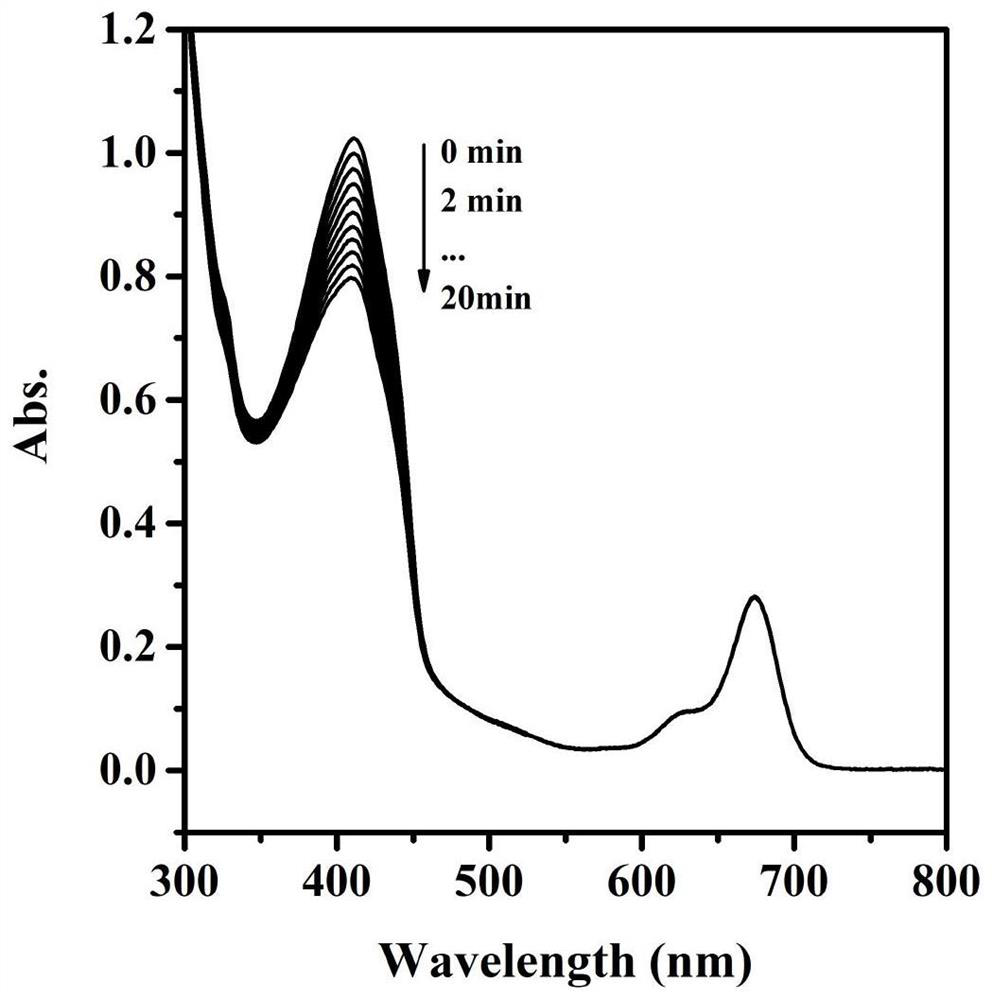
A kind of indole squaraine dye and its preparation method and application
An indole squaraine cyanine and dye technology, which is applied in the field of indole squaraine cyanine dye and its preparation, can solve the problems of low singlet oxygen quantum yield and the like, so as to solve the problem of low singlet oxygen quantum yield and improve production capacity , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] In this example, the compound of formula (III) was prepared by p-iodophenylhydrazine and 3-methyl-2-butanone.
[0060] Dissolve p-iodophenylhydrazine (1.17g, 5mmol) and 3-methyl-2-butanone (0.52g, 6mmol) in 30ml of glacial acetic acid, heat to reflux for 3h for the second reaction, cool to room temperature, and remove by rotary evaporation The solvent was extracted with ethyl acetate and water, and the extracted product was dried with anhydrous sodium sulfate, and the solvent was removed by rotary evaporation, and then purified with petroleum ether and ethyl acetate (volume ratio of 2:1) to obtain an orange oily liquid that was The compound with the structure shown in (III) was 1.09 g, the purity was 98%, and the yield was 76.5%.
[0061]
[0062] The nuclear magnetic data of the compound of structure shown in formula (III): 1 H NMR (400MHz, CDCl 3 ) δ 7.56–7.49 (m, 2H), 7.20 (d, J=7.9Hz, 1H), 2.16 (s, 3H), 1.18 (s, 6H).
Embodiment 2
[0064] In this example, the compound (SQ) with the structure represented by formula (II) was prepared by the compound represented by formula (III) and squarylium.
[0065] Under nitrogen protection, put squarylium (0.11g, 1mmol) in 10ml of n-butanol and 10ml of toluene in a Dean-Stark apparatus. After heating to reflux for 1h, squarylium was dissolved in a binary solvent system to obtain a reaction solution. The compound (0.57g, 2mmol) with the structure represented by formula (III) was dissolved in 5ml of n-butanol and 5ml of toluene, added dropwise to the reaction solution, and the reaction was stopped after the first reaction for 3h. Cool the first reaction product to precipitate a solid precipitate, add n-hexane to filter and wash the precipitate, then add dichloromethane and water to dissolve and extract the precipitate, dry it with anhydrous sodium sulfate, and rotate it to evaporate, then use a volume ratio of 1:1 The mixed solution of dichloromethane and petroleum ethe...
Embodiment 3
[0069] In this example, SQ-1 was prepared by the compound of formula (II) and aryl boronic acid.
[0070] The compound (324.2 mg, 0.5 mmol) of the structure shown in formula (II) and tetrakis (triphenylphosphine) palladium (57.7 mg, 0.05 mmol) were dissolved in 15 ml of ethylene glycol dimethyl ether, under nitrogen protection, After vacuuming, add phenylboronic acid (134.2mg, 1.1mmol) and saturated sodium bicarbonate solution (168.1mg, 2mmol), heat the temperature to 95°C for 4h Suzuki-Miyaura coupling reaction, Suzuki-Miyaura coupling reaction The product was washed three times with a saturated aqueous solution of ammonium chloride, the solvent was removed by rotary evaporation, extracted with dichloromethane and water, dried with anhydrous sodium sulfate, the solvent was removed by rotary evaporation, and finally the solvent was removed with a volume ratio of 2:1 petroleum ether The mixed solution with dichloromethane was purified by column chromatography to obtain a green ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com