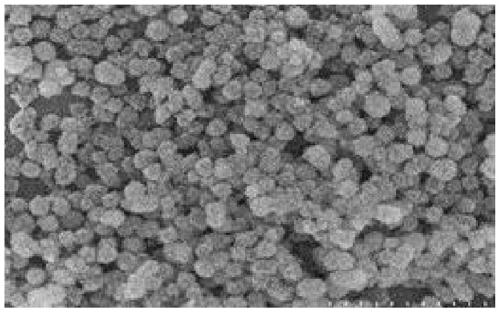
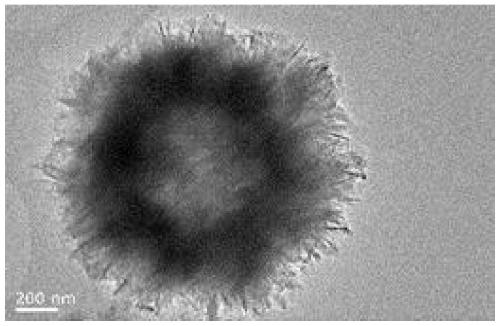
Preparation method of calcium phosphate microflowers
A micron flower and calcium phosphate technology, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve problems such as difficult large-scale production, high energy consumption, cumbersome synthesis process, etc., and achieve simple operation, short reaction time, shape stabilization effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
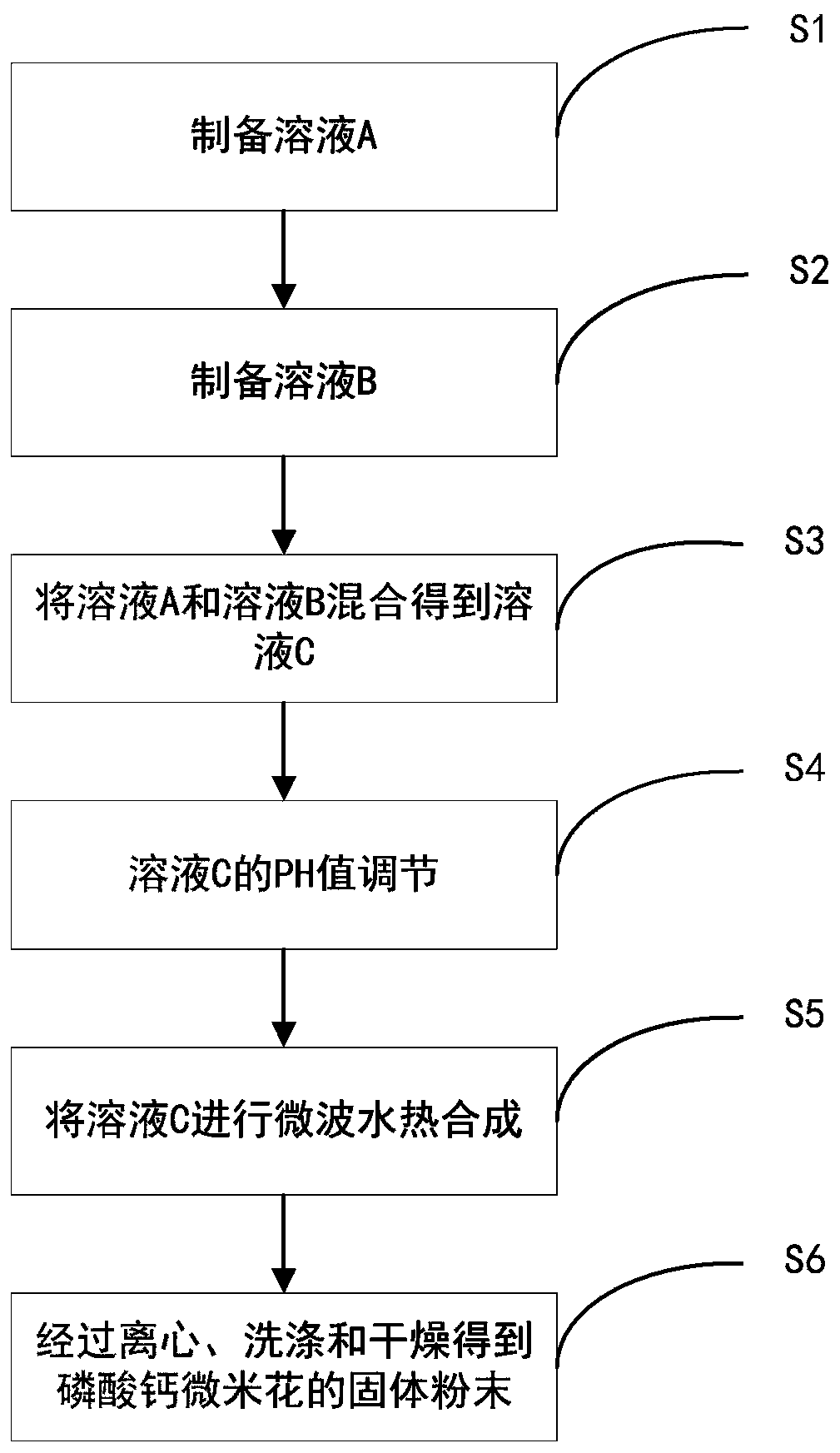
[0035] Such as figure 1 Shown, a kind of preparation method of calcium phosphate micron flower, comprises the steps:
[0036] S1: Dissolve calcium lactate in deionized water at a concentration of 0.001-100 mol / L under heating and stirring to obtain solution A. During specific implementation, the calcium lactate salt is selected from calcium lactate anhydrous, calcium lactate trihydrate or calcium lactate pentahydrate;
[0037] S2: under stirring, dissolve creatine phosphate disodium salt in deionized water according to the concentration of 0.001-100mol / L to obtain solution B;
[0038] S3: Wait until the solution A is cooled to room temperature, and mix the solution A with the solution B according to the molar ratio of calcium lactate and creatine phosphate (0.1-5.0):1 to obtain a mixed solution C;
[0039] S4: Slowly add an aqueous sodium hydroxide solution with a concentration of 2M under stirring, and adjust the pH value of the mixed solution to between 7-14;
[0040] S5:...
Embodiment 1
[0045] (1) Weigh 0.262g of calcium lactate pentahydrate, and dissolve calcium lactate in 100mL of deionized water under heating and stirring to obtain a solution A with a concentration of 12mM;
[0046] (2) Under stirring, dissolve 0.317g of creatine phosphate disodium salt in 100mL of deionized water to obtain a solution B with a concentration of 15mM;
[0047] (3) Wait until solution A is cooled to room temperature, and mix solution A and solution B according to the molar ratio of calcium lactate and creatine phosphate as 0.8:1 to obtain mixed solution C;
[0048] (4) Under stirring, slowly add a 2M sodium hydroxide aqueous solution to adjust the pH value of the mixed solution to 10;
[0049] (5) Transfer the mixed solution to a microwave reactor, and place it in the microwave reactor for microwave hydrothermal synthesis. The microwave reaction time is 10min, and the microwave reaction temperature is controlled at 120°C;
[0050] (6) After the reaction, the microwave react...
Embodiment 2
[0053] (1) Weigh 0.546g of calcium lactate pentahydrate, and dissolve calcium lactate in 100mL of deionized water under heating and stirring to obtain a solution A with a concentration of 24mM;
[0054] (2) Under stirring, dissolve 0.317g of creatine phosphate disodium salt in 100mL of deionized water to obtain a solution B with a concentration of 15mM;
[0055] (3) Wait until solution A is cooled to room temperature, and mix solution A and solution B according to the molar ratio of calcium lactate and creatine phosphate as 1.67:1 to obtain mixed solution C;
[0056](4) Under stirring, slowly add a 2M sodium hydroxide aqueous solution to adjust the pH value of the mixed solution to 10;
[0057] (5) Transfer the mixed solution to a microwave reactor, and place it in the microwave reactor for microwave hydrothermal synthesis. The microwave reaction time is 10min, and the microwave reaction temperature is controlled at 120°C;
[0058] (6) After the reaction, the microwave react...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com