A kind of preparation method of N-(indole-n-formyl)-alpha-aminoamide derivative
A kind of aminoamide, formyl technology, applied in the field of preparation of N--α-aminoamide derivatives, to achieve the effect of large application potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Weigh 33 mg (0.2 mmol) of N-indolecarboxylic acid and 36 mg (0.24 mmol) of p-nitrobenzaldehyde into a dry two-necked reaction flask, and inject 2 mL of solvent after being protected by an argon balloon (dichloromethane: methanol = 1:2) , followed by injecting 24 mg of aniline and 28 mg of benzyl isonitrile, reacting in an ice-water bath and stirring for 24 hours, TLC detected that the reaction was transformed completely, adding silica gel to mix the sample and separating by column chromatography (eluent was petroleum ether: ethyl acetate=5: 1) 74 mg of the product can be obtained, with a yield of 73%. The reaction formula is as follows:
[0041]
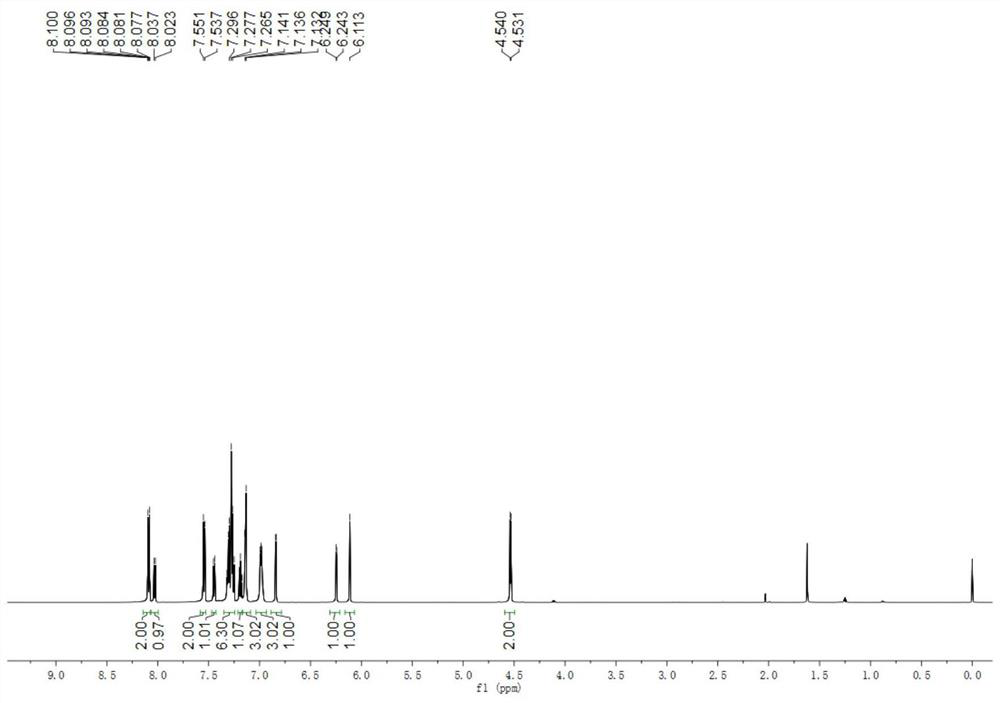
[0042] The physical properties and spectrogram data of the product are as follows: white solid, melting point 151–153°C; 1 HNMR (600MHz, CDCl 3 )δ8.13–8.07(m,2H),8.03(d,J=8.4Hz,1H),7.54(d,J=8.4Hz,2H),7.45(d,J=7.8Hz,1H),7.35– 7.24(m,6H),7.21–7.09(m,4H),7.04–6.93(m,3H),6.84(d,J=3.6Hz,1H),6.25(d,J=3.6Hz,1H),6.11 (s,1H),4.5...
Embodiment 2
[0044]Weigh 33 mg (0.2 mmol) of N-indolecarboxylic acid and 26 mg (0.24 mmol) of benzaldehyde into a dry two-necked reaction flask, inject 2 mL of solvent (dichloromethane: methanol = 1:2) after argon balloon protection, and then inject 24 mg of aniline and 28 mg of benzyl isocyanide were reacted in an ice-water bath and stirred for 24 hours. TLC detected that the reaction was completely converted. After adding silica gel to mix the sample, column chromatography separated (eluent: petroleum ether: ethyl acetate = 5: 1) 63 mg of the product was obtained, and the yield was 69%. The reaction formula is as follows:
[0045]
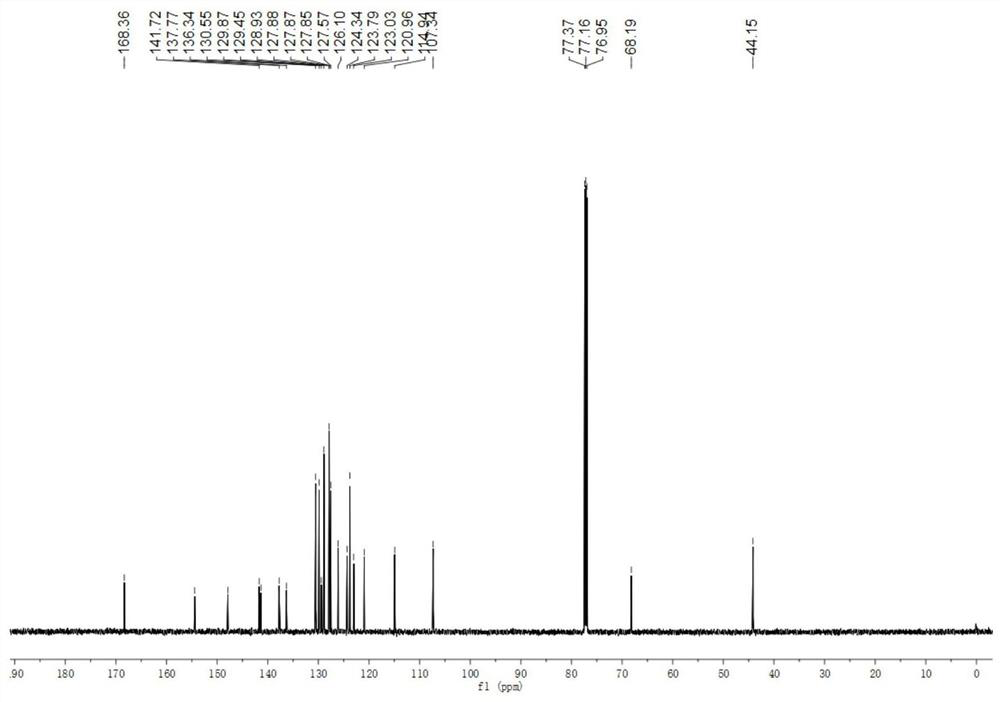
[0046] The physical properties and spectrogram data of the product are as follows: white solid, melting point 141–143°C; 1 H NMR (600MHz, CDCl 3 )δ8.13–8.04(m,1H),7.43(d,J=7.8Hz,1H),7.40–7.36(m,2H),7.32–7.23(m,9H),7.18–7.15(m,1H) ,7.12–7.06(m,3H),7.06–7.00(m,2H),6.87(d,J=3.6Hz,1H),6.50(t,J=5.4Hz,1H),6.22(d,J=3.6 Hz,1H),6.03(s,1H),4.62–4.48(m,2H); 13 C...
Embodiment 3
[0048] 33 mg (0.2 mmol) of N-indolecarboxylic acid and 49 mg (0.24 mmol) of 3-bromo-4-fluorobenzaldehyde were weighed into a dry two-necked reaction flask, and 2 mL of solvent (dichloromethane:methanol = 1:2), followed by injecting 24 mg of aniline and 28 mg of benzyl isonitrile, reacted and stirred for 24 hours in an ice-water bath, TLC detected that the reaction had transformed completely, added silica gel to mix the sample and separated by column chromatography (eluent was petroleum ether: ethyl acetate Ester=5:1) 89 mg of the product can be obtained, and the yield is 80%. The reaction formula is as follows:
[0049]
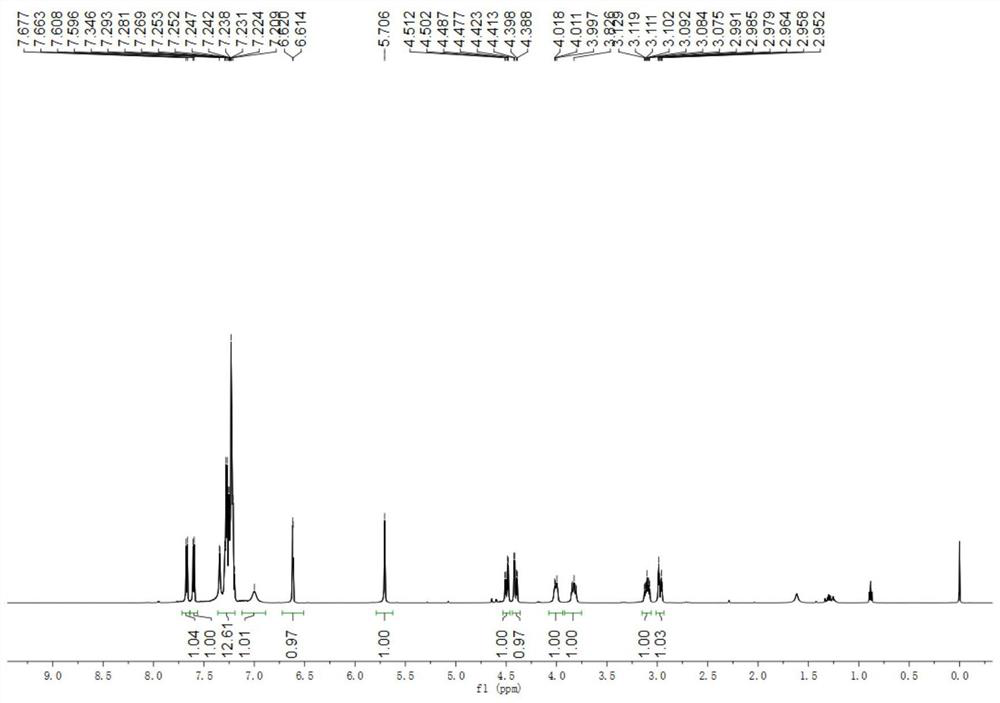
[0050] The physical properties and spectrogram data of the product are as follows: white solid, melting point 134–136°C; 1 H NMR (400MHz, CDCl 3 )δ8.05(d,J=8.4Hz,1H),7.56(dd,J 1 =6.4Hz,J 2 =2.0Hz,1H),7.44(d,J=7.6Hz,1H),7.34–7.23(m,7H),7.21–7.08(m,4H),7.03–6.92(m,3H),6.83(d, J=3.6Hz, 1H), 6.73(t, J=6.0Hz, 1H), 6.22(d, J=3.6Hz, 1H), 6.00(s, 1H), 4.52(d,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com