Quinoline compound and synthetic method and application thereof
A technology of compounds and quinolines, which is applied in the field of quinolines and their synthesis, can solve the problems of complex synthesis process, few types, and insignificant weed effect, and achieve good weeding effect, good effect, and simple and easy synthesis method line effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
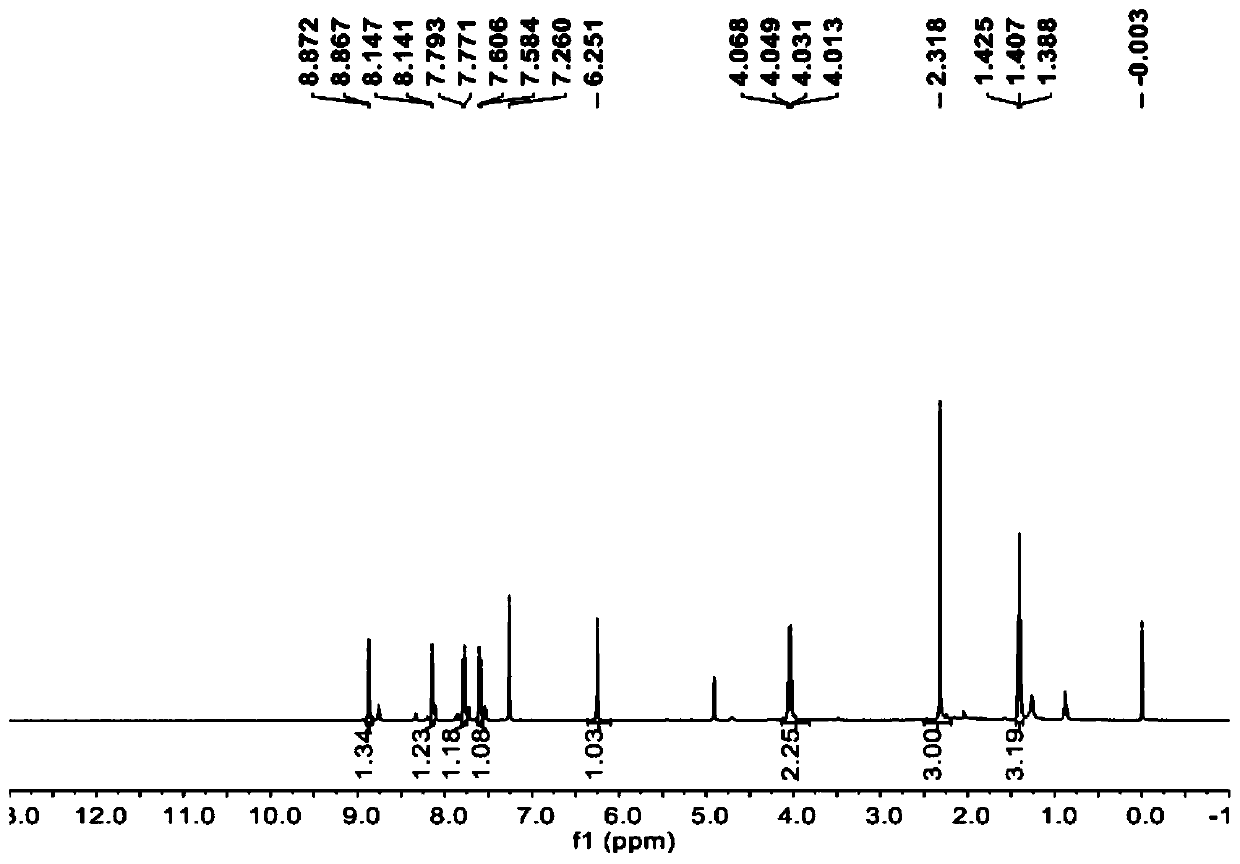
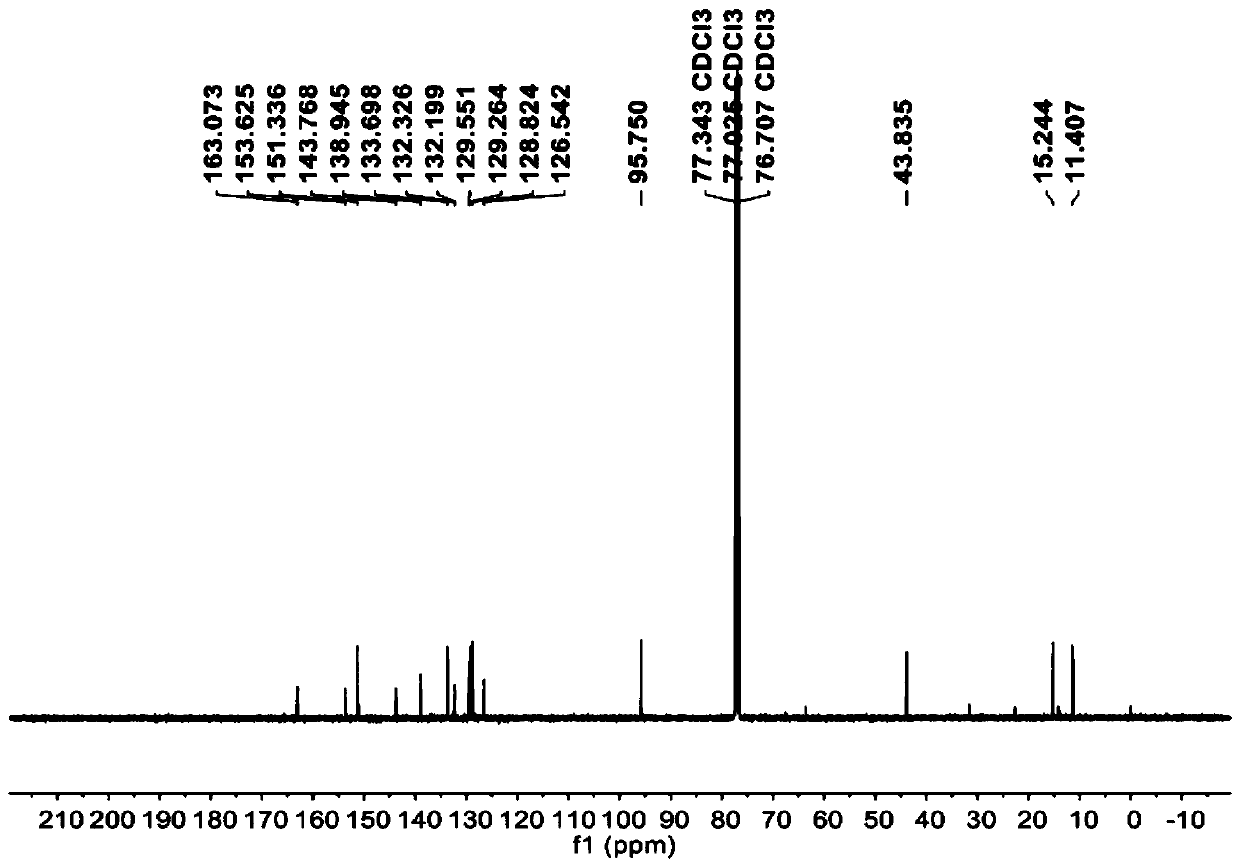
[0029] 10mmol substituent R 1 Be the compound N (its structural formula is as follows) of Cl atom and the acid chloride SOCl of 80mmol 2 Reagent, dissolved in 150mL of toluene solvent, reacted for 12h, cooled, and the solvent and excess SOCl were evaporated under reduced pressure 2 , to get the substituent R 1 The intermediate M (its structural formula is as follows) of Cl is ready for use.
[0030] 10 mmol of substituent R 2 The compound L (its structural formula is as follows) that is ethyl, the calcium hydroxide of 17mmol, the calcium oxide of 3mmol are added in the 250mL two-necked flask, after adding the acetonitrile of 80mL, heating and stirring and refluxing for 0.5h, will be dissolved in the acetonitrile solvent of 20mL 10 mmol of intermediate M was added dropwise to the reaction system, and the reaction was continued under reflux for 12 hours. After the reaction was completed, it was cooled and poured into 200 mL of ice water, adjusted to pH=4-5 with hydrochloric a...
Embodiment 2
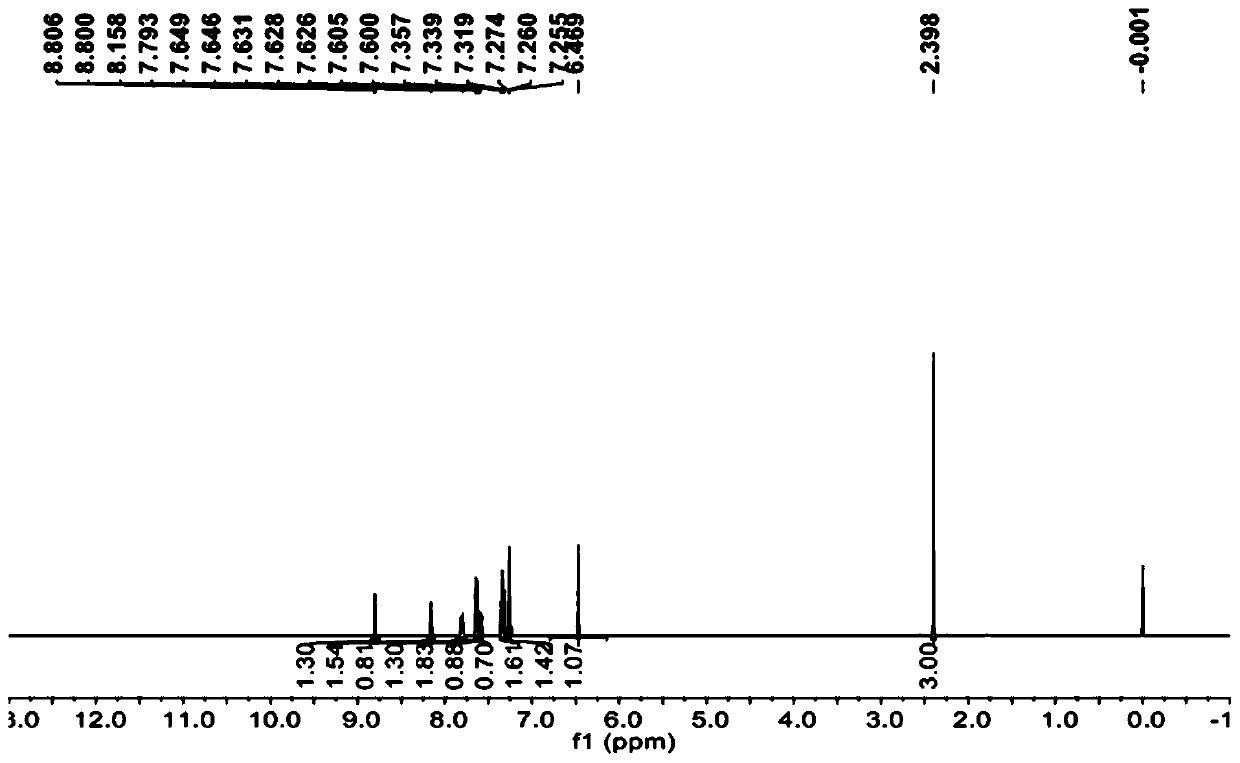
[0039] 10mmol substituent R 1 Be the compound N of nitro (its structural formula is as follows) and the acid chloride POCl of 80mmol 3 Reagent, dissolved in 150mL of toluene solvent, reacted for 12h, cooled, and the solvent and excess SOCl were evaporated under reduced pressure 2 , to get the substituent R 1 The intermediate M (its structural formula is as follows) of Cl is ready for use.
[0040] 12 mmol of substituent R2 Phenyl compound L (its structural formula is as follows), 17mmol of magnesium hydroxide, and 3mmol of magnesium oxide were added to a 250mL two-necked flask, and 80mL of 1,4-dioxane was added, heated and stirred under reflux for 1 hour, and the dissolved 10mmol of intermediate M in 20mL of 1,4-dioxane solvent was added dropwise to the reaction system, and after reflux reaction for 24h (the disappearance of the raw material point was monitored by thin-layer chromatography), it was cooled and poured into 200mL of ice water, and used Sulfuric acid was used t...
Embodiment 3
[0049] 10mmol substituent R 1 Be the compound N (its structural formula is as follows) of Cl atom and the acid chloride SOCl of 80mmol 2 Reagents, dissolved in 150mL of tetrahydrofuran solvent, reacted for 12h, cooled, and the solvent and excess SOCl were evaporated under reduced pressure 2 , to get the substituent R 1 The intermediate M (its structural formula is as follows) of Cl is ready for use.
[0050] 15mmol substituent is R 2 Benzyl compound L (its structural formula is as follows), 17mmol of calcium hydroxide and 3mmol of magnesium oxide were added to a 250mL two-necked flask, and 100mL of acetonitrile was added, heated and stirred under reflux for 0.5h, and then dissolved in 20mL of acetonitrile solvent 10mmol of intermediate M was added dropwise to the reaction system, and refluxed for 24h. Cool and pour into 200 mL of ice water, adjust the pH to 4-5 with sulfuric acid, and filter to obtain a solid.
[0051] Dissolve the solid in chloroform, add water for extra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com