Pharmaceutical composition with anti-asthmatic activity and application thereof
A composition and drug technology, applied in the direction of drug combination, organic active ingredients, medical preparations containing active ingredients, etc., can solve problems such as easy bleeding, poor wound healing, purple streaks, etc., and achieve enhanced quality effects, good mast cells Stabilizing and anti-inflammatory effects, dose reduction effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
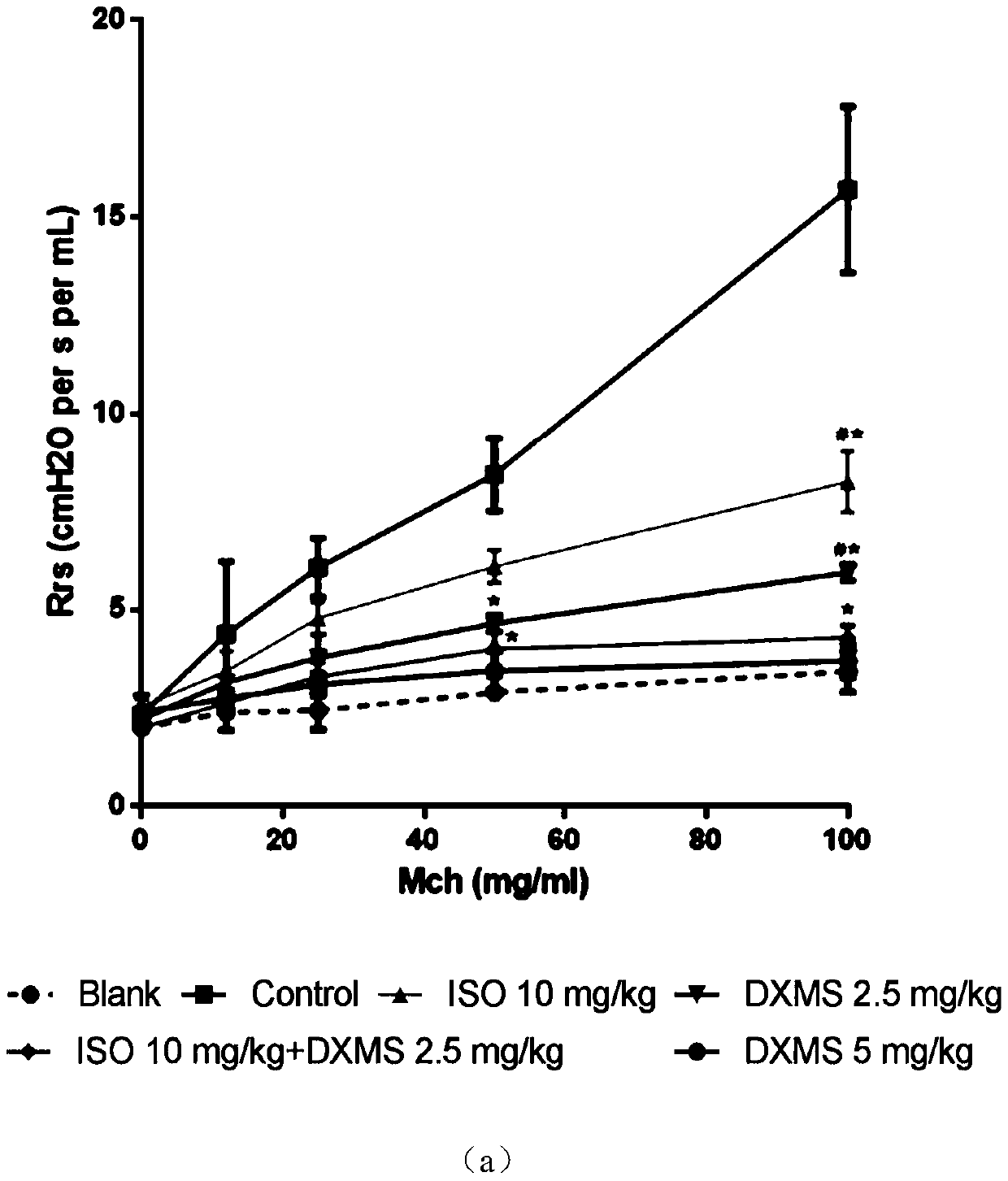
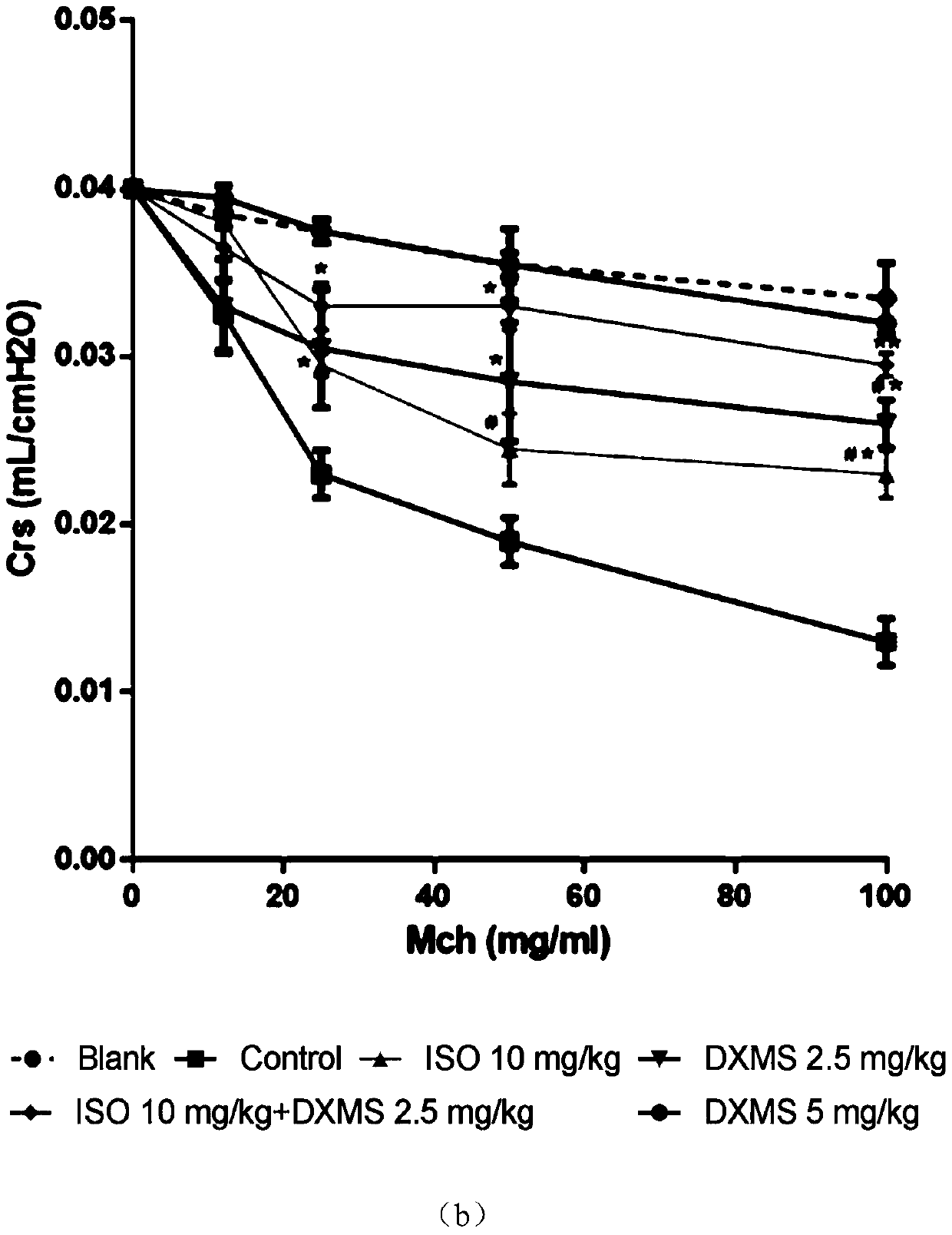
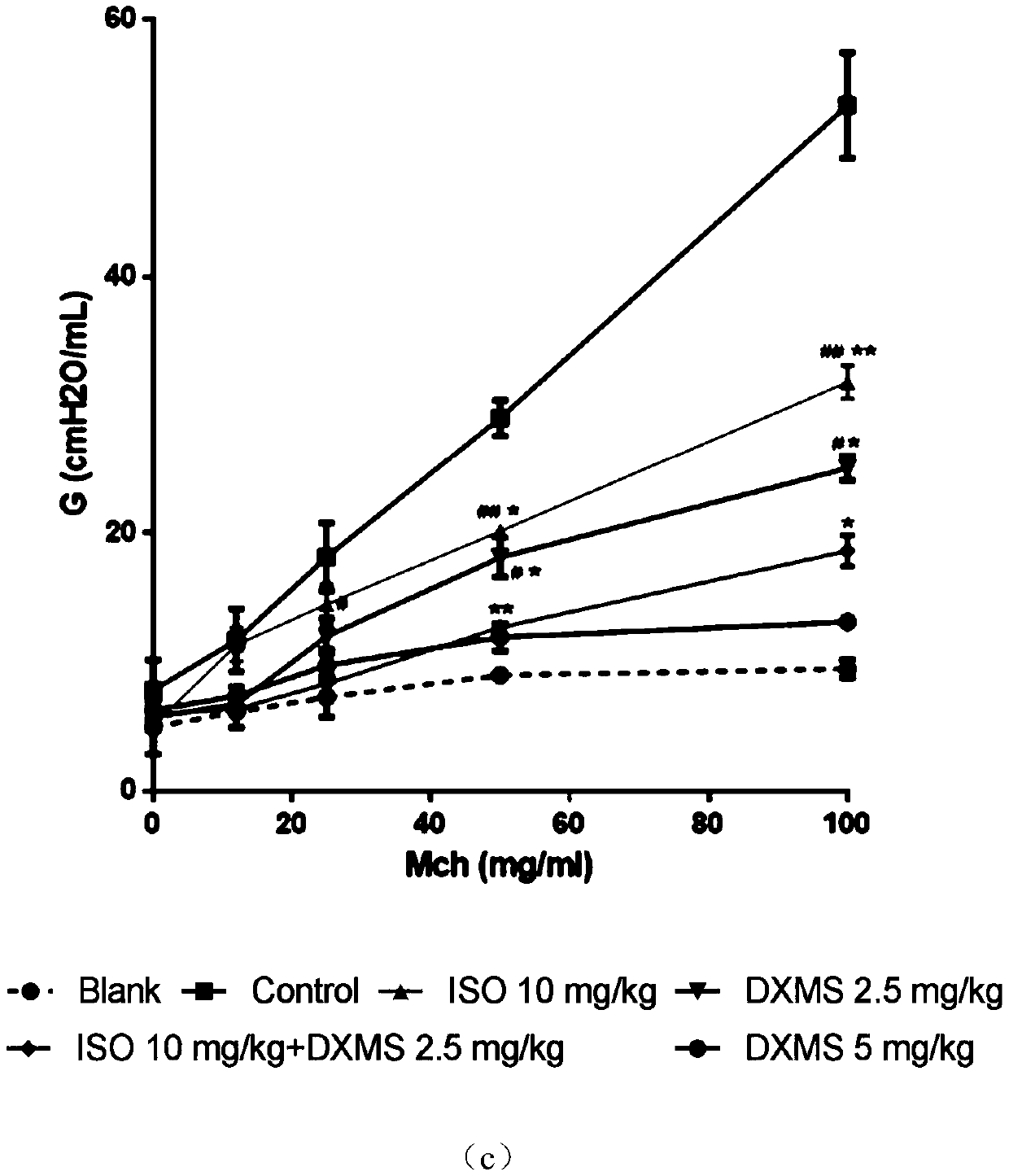
[0031] The mouse asthma model established by ovalbumin (OVA) was used to compare the therapeutic effects of dexamethasone and isoimperatorin alone and in combination on allergic asthma.
[0032] Get C57 / BL6 mouse, divide into 7 groups randomly, namely blank control group (PBS), model control group (PBS), isoimperatorin group (10mg / kg), dexamethasone low concentration group (2.5mg / kg). kg), dexamethasone high concentration group (5mg / kg) and combined administration group (2.5mg / kg dexamethasone+10mg / kg isoimperatorin).
[0033] On the 1st, 3rd, 5th and 7th days, the mice in each group were intraperitoneally injected with 0.5 mg / kg OVA to sensitize the mice, and the blank control group was injected with normal saline. On the 21st, 23rd, 25th, 27th and 29th day, the mice were placed in a closed container, and 1% OVA was inhaled to induce asthma attack, once a day, 30 minutes each time. The blank control group inhaled normal saline by nebulization.
[0034] Intragastric administ...
Embodiment 2
[0040] To compare the effects of long-term single administration and combined administration of dexamethasone and isoimperatorin on the femoral head of mice.
[0041]Get C57 / BL6 mouse, be divided into 5 groups at random, i.e. blank control group, isoimperatorin group (10mg / kg), dexamethasone low concentration group (2.5mg / kg), dexamethasone high concentration group ( 5mg / kg) and combined administration group (10mg / kg isoimperatorin+2.5mg / kg dexamethasone).
[0042] Continuous intragastric administration for 30 days.
[0043] After the administration, the femoral heads on both sides of the mice were removed, and the surrounding soft tissues were removed and placed in place for HE staining after decalcification. Observe the changes of bone trabeculae, bone cells, and adipocytes in the medullary cavity.
[0044] from figure 2 In (a), (b), and (c), it can be seen that the cartilage layer of the femoral head in the blank control group is thicker, and the bone trabeculae are fil...
Embodiment 3
[0046] A pharmaceutical composition with anti-asthma activity, comprising dexamethasone and isoimperatorin in a mass ratio of 1:20.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com