Controllable synthesis method for stereotactic polymethylmethacrylate
A technology of polymethyl methacrylate and methyl methacrylate, which is applied in the field of polymer science, can solve the problems of uncontrollable chain transfer and side reactions, high reactivity and low stability of the growth group, and achieve a synthetic route Clear and actionable, simple to operate, and easy-to-control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0027] The present invention will be further described in detail below in conjunction with the accompanying drawings and specific embodiments.
[0028] The controllable synthesis method of a kind of stereoregular polymethyl methacrylate of the present invention, concrete steps are as follows:
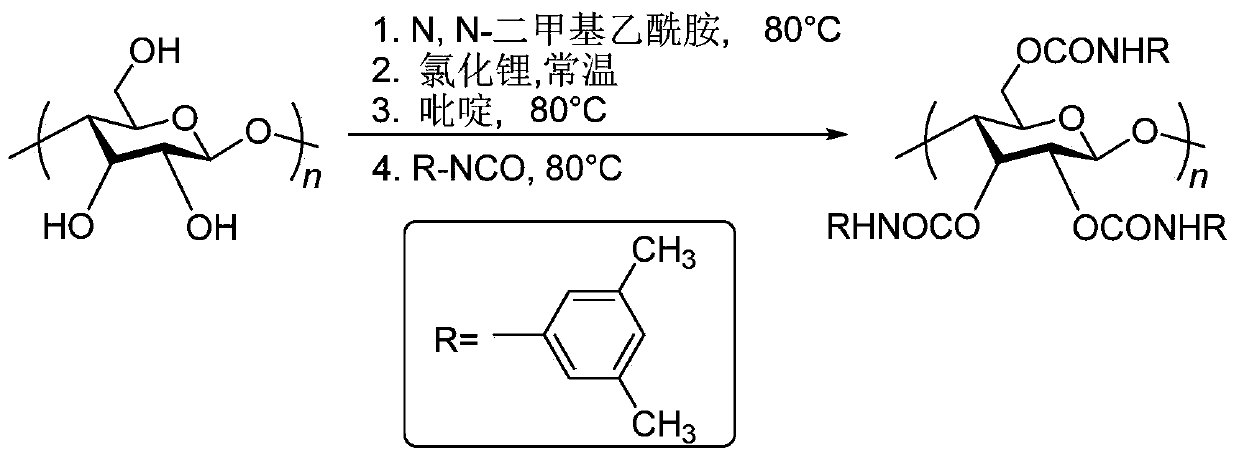
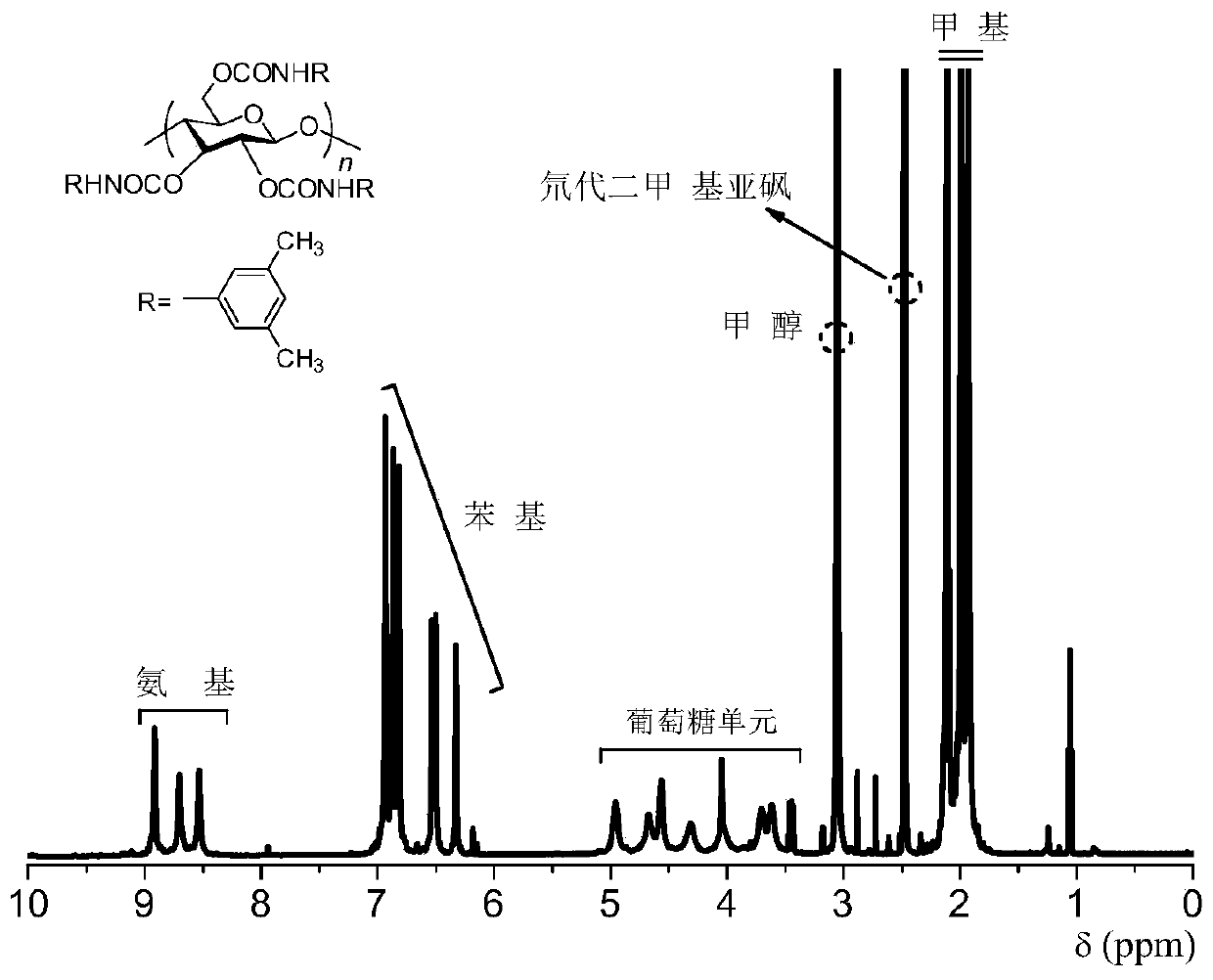
[0029] 1) Take microcrystalline cellulose and vacuum-dry it at high temperature for 4 hours, then stir and reflux in anhydrous N,N-dimethylacetamide for 12 hours; add an appropriate amount of lithium chloride after cooling to room temperature; continue to stir for 2 hours, then heat up again , add anhydrous pyridine, reflux for 6 hours, add excess 3,5-dimethylphenylisocyanate, react for 13 hours, stop the reaction; cool to room temperature, add methanol to settle, filter and wash, vacuum dry at 60°C to constant weight, and produce The rate is 95%.
[0030] 2), weigh the corresponding mass of the product obtained in 1) according to the 17 groups of different ratios set with the methyl m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com