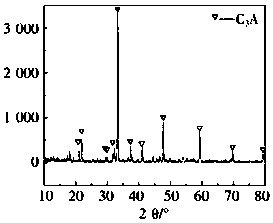
Method of preparing high-purity tricalcium aluminate quickly
A tricalcium aluminate, high-purity technology, applied in the preparation of calcium aluminate, alkaline earth metal aluminate/alumina/aluminum hydroxide, sustainable manufacturing/processing, etc., can solve the problem of high energy consumption and high firing temperature , long firing cycle and other problems, to achieve the effect of reducing preparation conditions, good synthesis effect, and improving long cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Purpose: To prepare 15.00g of tricalcium aluminate
[0035] The oxide form 3CaO Al according to the chemical formula of tricalcium aluminate 2 o 3 , the calculated CaO content is 62.27%, Al 2 o 3 The content of CaO is 37.73%. According to 15.00g of the sample to be prepared, the calculated content of CaO in the sample should be 9.34g. According to the conservation of calcium element mass, the calculated amount of used analytically pure calcium nitrate tetrahydrate is 39.32g.
[0036] Press N(Ca(NO 3 ) 2 4H 2 O):N(Al(OH) 3 Colloid) = 3:1, weighed 4.33g of aluminum hydroxide; according to the calcium ion concentration of 1.9mol / L, the mass of deionized water was calculated to be 89.47g, and the corresponding mass of deionized water was weighed.
[0037] The calcium nitrate tetrahydrate that weighs, aluminum hydroxide and deionized water are packed in 300ml Erlenmeyer flask, put into magnetic stirrer, be fixed on the magnetic stirrer and heat, Erlenmeyer flask is se...
Embodiment 2
[0042] Purpose: To prepare 20.00g of tricalcium aluminate
[0043] The oxide form 3CaO Al according to the chemical formula of tricalcium aluminate 2 o 3 , the calculated CaO content is 62.27%, Al 2 o 3 The content of CaO is 37.73%. According to 20.00g of the sample to be prepared, the calculated content of CaO in the sample should be 12.45g. According to the conservation of calcium element mass, the calculated amount of used analytically pure calcium nitrate tetrahydrate is 52.41g.
[0044] Press N(Ca(NO 3 ) 2 4H 2O):N(Al(OH) 3 colloid)=3:1, weighed 5.77g aluminum hydroxide; according to the calcium ion concentration of 1.8mol / L, the calculated mass of deionized water was 122.22g, and weighed the corresponding mass of deionized water.
[0045] The calcium nitrate tetrahydrate that weighs, aluminum hydroxide and deionized water are packed in 300ml Erlenmeyer flask, put into magnetic stirrer, be fixed on the magnetic stirrer and heat, Erlenmeyer flask is sealed with plas...
Embodiment 3
[0050] Purpose: To prepare 25.00g of tricalcium aluminate
[0051] The oxide form 3CaO Al according to the chemical formula of tricalcium aluminate 2 o 3 , the calculated CaO content is 62.27%, Al 2 o 3 The content of CaO is 37.73%. According to 25.00g of the sample to be prepared, the calculated content of CaO in the sample should be 15.57g. According to the conservation of calcium element mass, the calculated amount of used analytically pure calcium nitrate tetrahydrate is 65.55g.
[0052] Press N(Ca(NO 3 ) 2 4H 2 O):N(Al(OH) 3 colloid)=3:1, weighed 7.22g of aluminum hydroxide; according to the calcium ion concentration of 2.0mol / L, the calculated mass of deionized water was 140g, and weighed the corresponding mass of deionized water.
[0053] The calcium nitrate tetrahydrate that weighs, aluminum hydroxide and deionized water are packed in 300ml Erlenmeyer flask, put into magnetic stirrer, be fixed on the magnetic stirrer and heat, Erlenmeyer flask is sealed with pla...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com