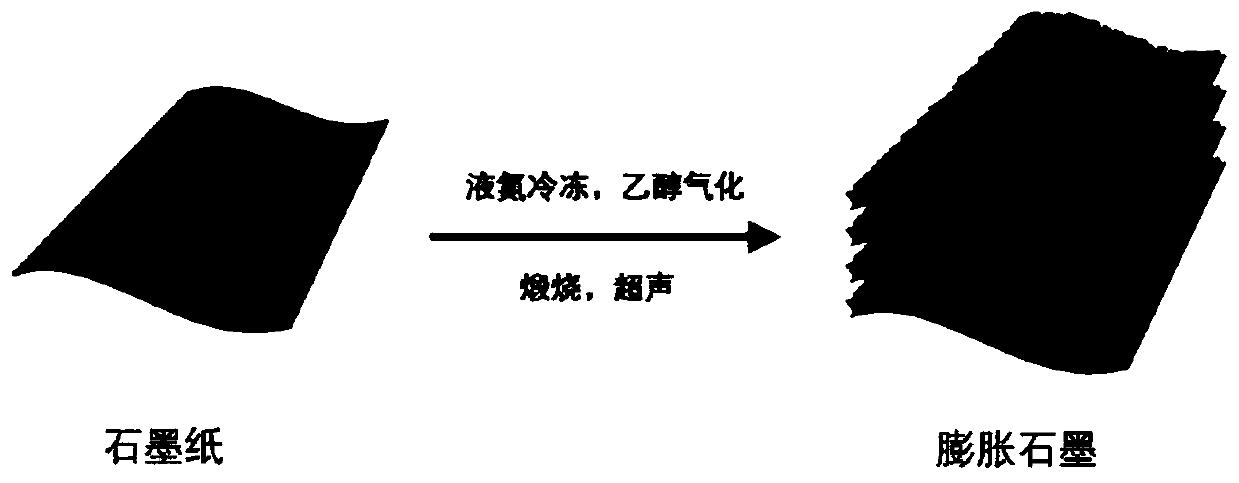
Expanded graphite electrode for electrochemical removal of heavy metal ion in wastewater and preparation method and application of expanded graphite electrode
A technology of heavy metal ions and expanded graphite, applied in chemical instruments and methods, water pollutants, water/sewage treatment, etc., can solve the problems of lack of active sites on the electrode surface, small specific surface area, poor metal ion reduction performance, etc. The preparation method is convenient, the material is simple, and the price is low.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 Preparation of expanded graphite electrode for treatment of electroplating wastewater containing hexavalent chromium
[0023] Electroplating wastewater contains 187mg / L of hexavalent chromium, COD=50mg / L, pH=3.5. The reactor is a typical double-chamber electrolytic cell. The reactor is composed of two rectangular reaction chambers with a volume of 220ml. The length, width and height are respectively: 5cm; 4cm; 11cm. The effective volume used in the experiment is 200ml. The outer wall of the reactor is made of plexiglass. The area between the two reaction chambers is 45cm 2 The proton exchange membrane is physically separated. The two reaction chambers are connected by flanges. In order to prevent leakage, rubber rings are used to seal the flanges and the proton exchange membranes. The opening above the anode chamber is sealed with a rubber plug, and 3 openings are reserved on the rubber plug for wires, sampling ports, etc. After the reactor is built, the ...
Embodiment 2
[0026] Example 2 Preparation of Expanded Graphite Electrode for Copper-containing Wastewater Treatment
[0027] The waste water contains 78mg / L of copper ions, COD=20mg / L, pH=6.3. The reaction device is a typical double-chamber electrolytic cell. The reactor is composed of two rectangular reaction chambers with a volume of 220ml. The length, width and height are respectively: 5cm; 4cm; 11cm. The effective volume used in the experiment is 200ml. The outer wall of the reactor is made of plexiglass. The area between the two reaction chambers is 45cm 2 The proton exchange membrane is physically separated. The two reaction chambers are connected by flanges. In order to prevent leakage, rubber rings are used to seal the flanges and the proton exchange membranes. The opening above the anode chamber is sealed with a rubber plug, and 3 openings are reserved on the rubber plug for wires, sampling ports, etc. After the reactor is built, the gap is sealed with high-density sealant. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com