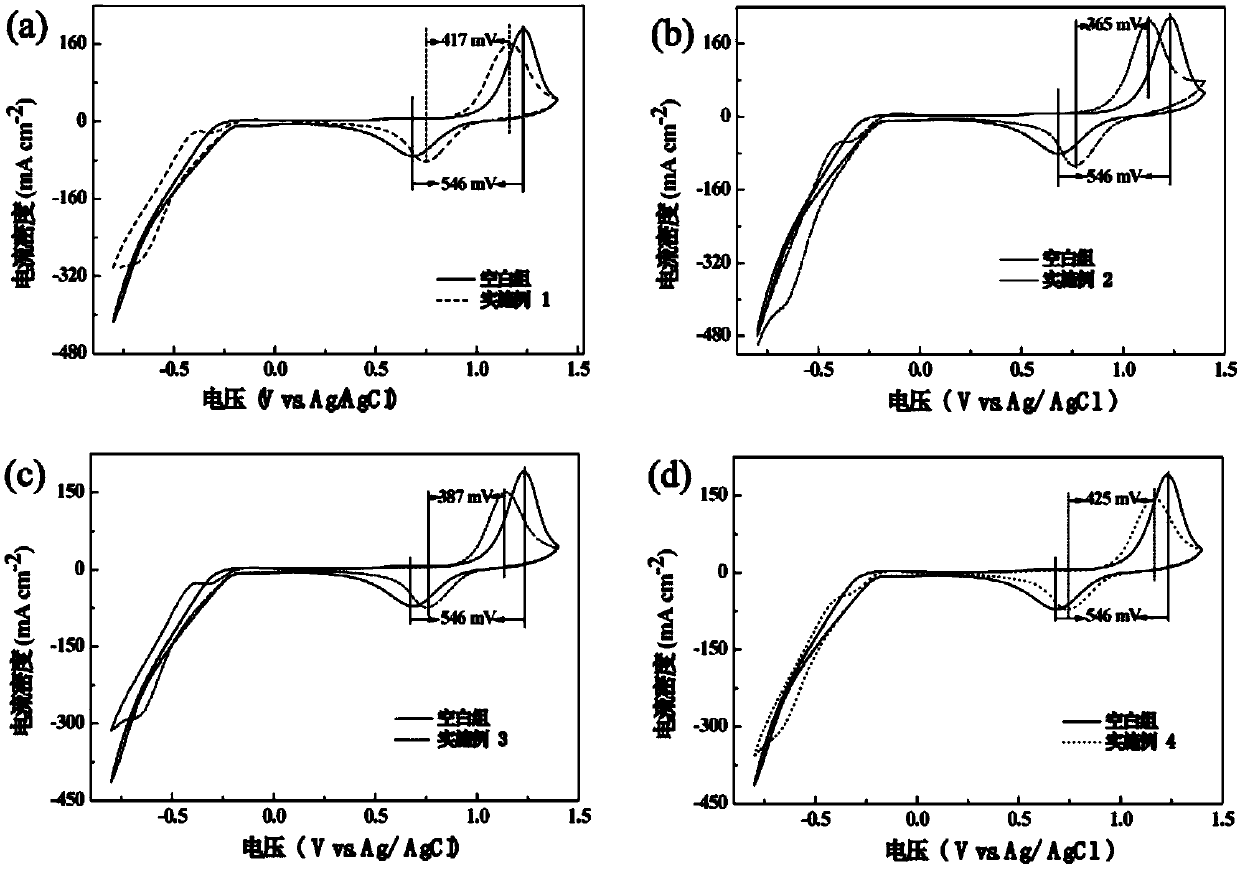
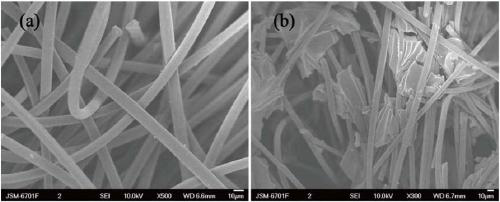
Modified carbon material and nitrogen-rich liquid flow battery electrode prepared from modified carbon material
A technology of carbon materials and modified carbon, which is applied in battery electrodes, fuel cells, regenerative fuel cells, etc., can solve the problems of limiting large-scale application and single catalytic activity function, and achieves simple and easy preparation methods and high specific surface area , good catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Add 25mL formaldehyde solution (2.5mL 37wt% formaldehyde solution dispersed in 22.5mL water) into the homemade 250mL reactor, cut 3*4cm 2 The commercialized graphite felt material was immersed in the solution; 0.6g urea and 0.63g melamine were respectively dispersed in a small amount of deionized water and ultrasonicated for 10min to obtain a homogeneous liquid. Pour the urea solution and the melamine solution into the reactor respectively, and the volume of the solution in the reactor is between 60mL-80mL, and does not change with the concentration of the reactants. Under the condition of magnetic stirring, the reaction temperature is controlled at 70°C, and KOH solution is added dropwise to adjust its pH to 8.5. Under alkaline conditions, urea and melamine are polymerized with formaldehyde respectively to form methylol urea and methylol melamine. Control the reaction time for 60 minutes, and test the pH change of the solution. After the pH is stable, add HCl dropwise ...
Embodiment 2
[0047] Add 25mL formaldehyde aqueous solution (4.16mL 37wt% formaldehyde aqueous solution dispersed in 20.84mL water) to the self-made 250mL reactor, and cut 3*4cm 2 The commercialized graphite felt material was immersed in the solution; 1.0g urea and 1.05g melamine were respectively dispersed in a small amount of deionized water, and the homogeneous liquid was obtained by ultrasonication for 10min. Pour the urea solution and the melamine solution into the reactor respectively, and the volume of the solution in the reactor is between 60mL-80mL, and does not change with the concentration of the reactants. Under the condition of magnetic stirring, the reaction temperature is controlled at 70°C, and KOH solution is added dropwise to adjust its pH to 8.5. Under alkaline conditions, urea and melamine are polymerized with formaldehyde respectively to form methylol urea and methylol melamine. Control the reaction time for 60 minutes, and test the pH change of the solution. After the ...
Embodiment 3
[0051] Add 25mL formaldehyde solution (3.125mL 37wt% formaldehyde solution dispersed in 21.875mL water) into the homemade 250mL reactor, and cut 3*4cm 2 The commercialized graphite felt material was immersed in the solution; 0.75g urea and 0.7875g melamine were respectively dispersed in a small amount of deionized water and ultrasonicated for 10min to obtain a homogeneous liquid. Pour the urea solution and the melamine solution into the reactor respectively, and the volume of the solution in the reactor is between 60mL-80mL, and does not change with the concentration of the reactants. Under the condition of magnetic stirring, the reaction temperature is controlled at 70°C, and KOH solution is added dropwise to adjust its pH to 8.5. Under alkaline conditions, urea and melamine are polymerized with formaldehyde respectively to form methylol urea and methylol melamine. Control the reaction time for 60 minutes, and test the pH change of the solution. After the pH is stable, add HC...
PUM
| Property | Measurement | Unit |
|---|---|---|
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com