Rotigotine-containing percutaneous absorption type patch
A patch and transdermal technology, which is applied in the field of lining film and anti-mucosal film, can solve problems such as difficulty in ensuring drug stability, and achieve the effect of increasing skin adhesion and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-5
[0071] Embodiment 1-5, and comparative example 1-9
[0072] Manufacture of transdermal patch
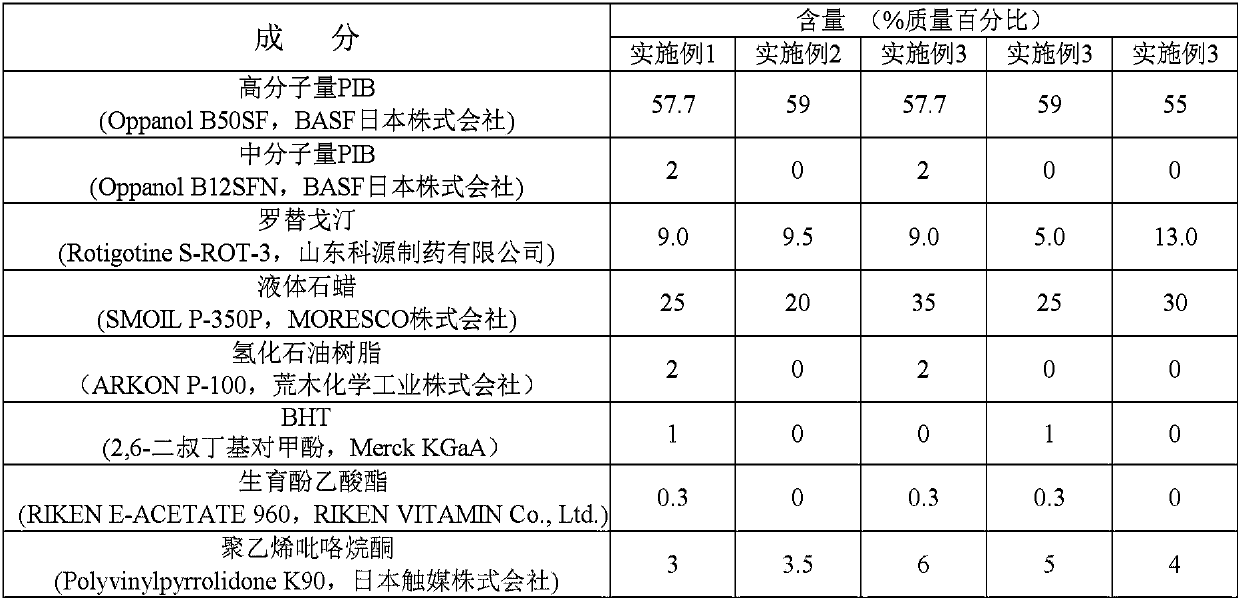
[0073] According to the content ratios listed in Tables 1 and 2, appropriate high-molecular-weight PIB, medium-molecular-weight PIB, alicyclic saturated hydrocarbon, liquid paraffin, etc. were mixed in cyclohexane and stirred to dissolve. Then add the ethanol solution of polyvinylpyrrolidone and / or methylpyrrolidone to the mixture, then further add the rotigotine solution obtained by mixing the tocopherol acetate and the ethyl acetate solution of rotigotine, stir and mix to obtain homogeneous solution. Use a test coater (TransCoat, manufactured by Cosmet Pharmaceutical Co., Ltd., Japan) to prepare a colloid with a drug-containing layer of 50 μm in thickness after evaporating the organic solvent, and make it on a release film (silicon resin-treated PET film, Fujimori Industrial Co., Ltd., Japan) manufacturing) spread evenly. After applying the drug-containing solution, the organic ...
Embodiment 6~10 and 11
[0116] Embodiment 6~10 and 11, comparative example 10~11
[0117] Comparative test of chemical grades of polyvinylpyrrolidone
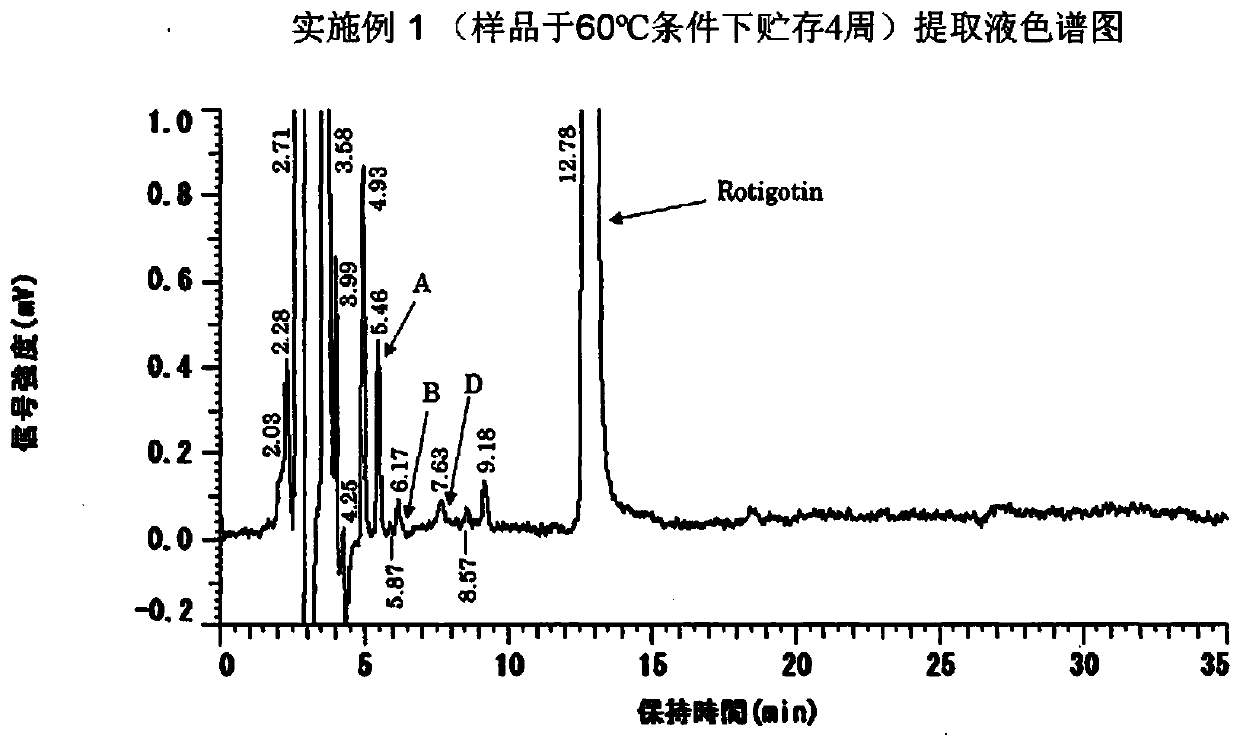
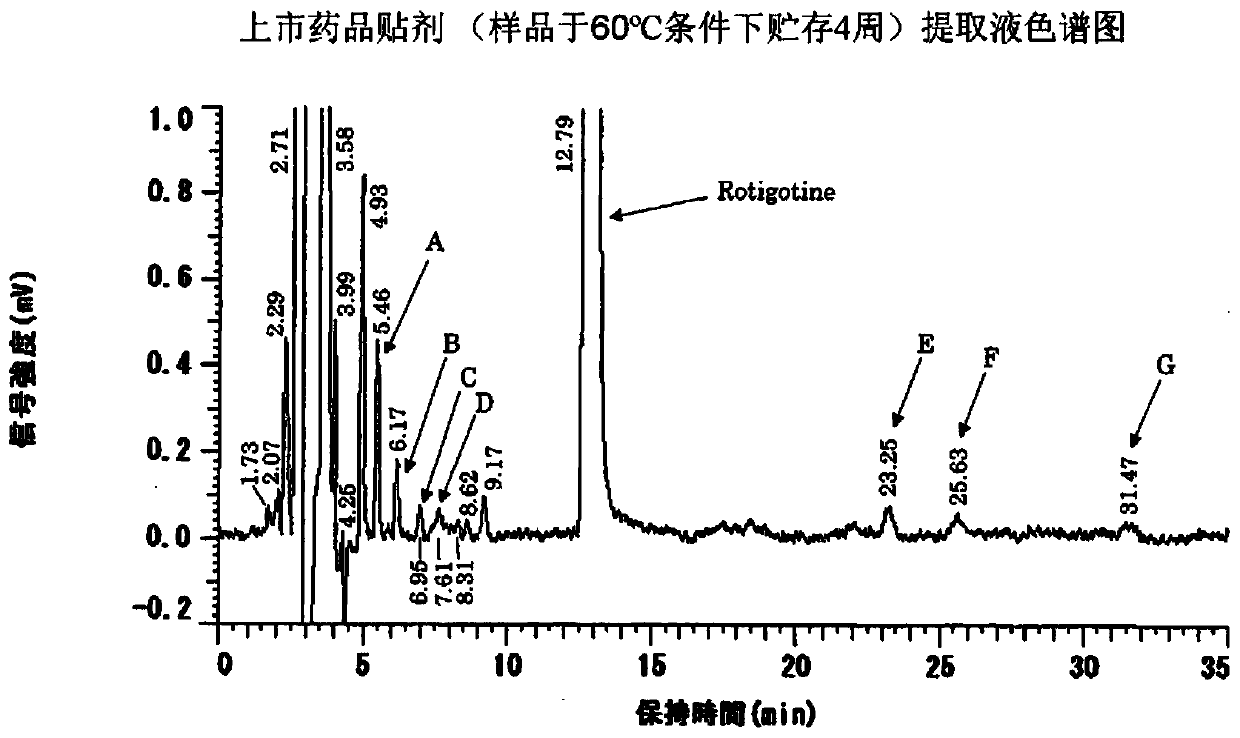
[0118] In the following Examples 6 to 10 and Comparative Examples 10 and 11, various grades of polyvinylpyrrolidone were used, and through the aforementioned drug stability experiments, it was observed that the drug stability significantly changed depending on the chemical grade of polyvinylpyrrolidone. Measure the stability of the drug after storing the preparation at high temperature, and observe its decomposition product peak (peak E with a retention time of about 23 minutes, see figure 2 ), the area of peak E measured by HPLC is divided by the peak area of rotigotine (i.e. the content percentage of the decomposition product in the main drug) to measure the stability of the preparation. The analysis method is the same as the aforementioned drug stability test. When the formulation was stored under heating at 60° C. for one week, and the rati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com