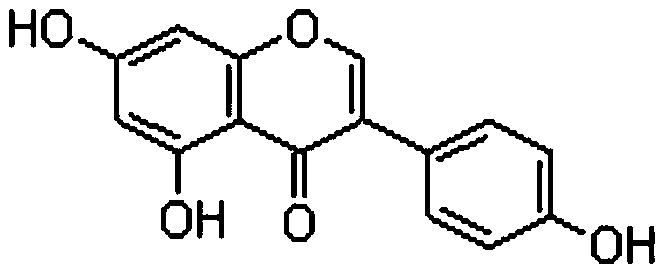
Use of genistein in preparation of drugs to prevent or treat African swine fever
A technology of genistein and African swine fever, applied in the field of medicine, can solve the problems of legacy antibiotics, poor oral treatment effect, easy recurrence of disease, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
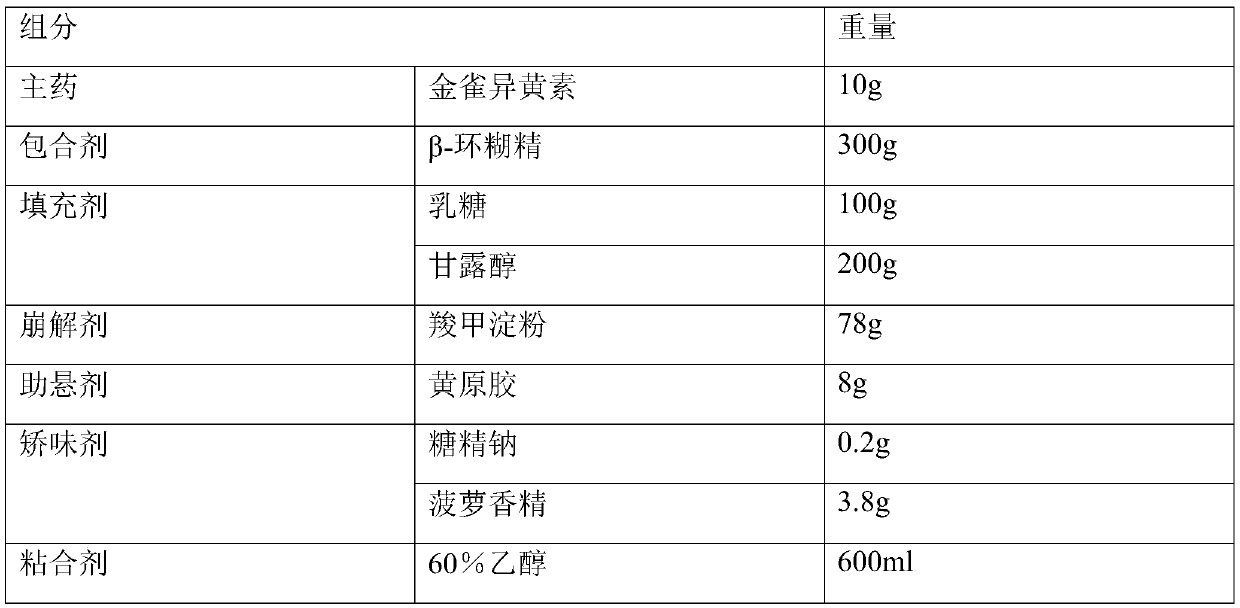
[0020] Embodiment 1: Preparation of genistein dry suspension
[0021] prescribing information
[0022]
[0023] Preparation:
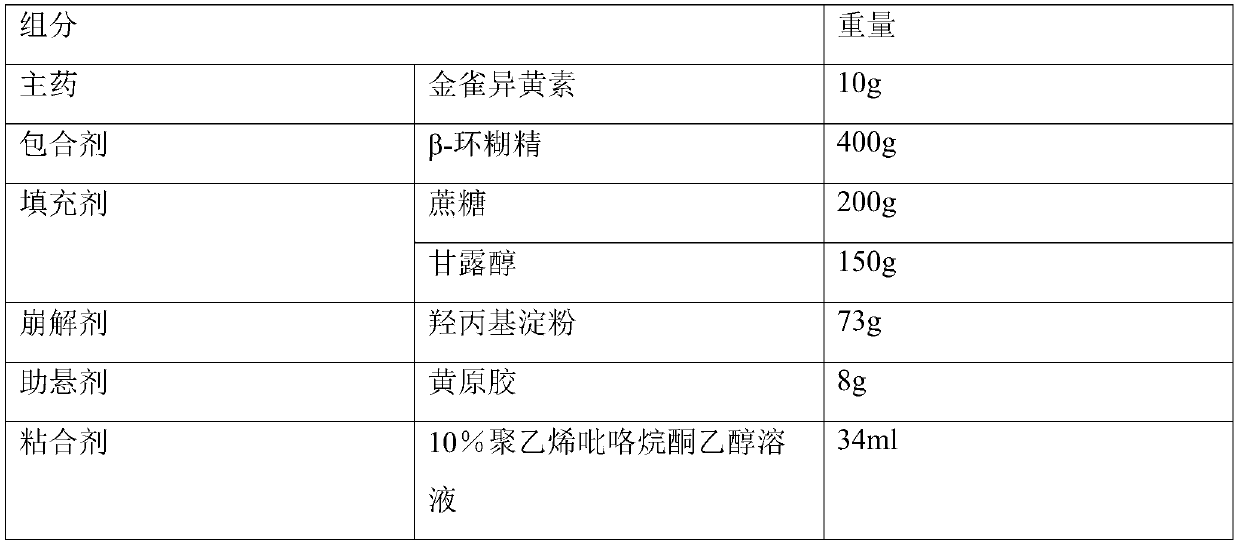
[0024] Add genistein to 300ml ethanol, stir to dissolve, then add β-cyclodextrin and magnetically stir, then concentrate, dry, pulverize, and pass through a 120-mesh sieve; 100 mesh sieve, spare. Weigh and mix genistein inclusion compound, lactose, mannitol, sodium starch glycolate and xanthan gum according to the prescription amount, stir for more than 30 minutes, and mix evenly. Dissolve the prescribed amount of sodium saccharin and pineapple flavor in the remaining ethanol to make a binder and make a soft material. Granulate with 20-mesh sieve, dry at 55-60°C; granulate with 18-mesh sieve. Packed in composite film bags or bottles.
Embodiment 2
[0025] Embodiment 2: Preparation of genistein dry suspension
[0026] prescribing information
[0027]
[0028] Preparation:
[0029] Add genistein to 720ml ethanol, stir to dissolve, then add hydroxypropyl-β-cyclodextrin and magnetically stir, then concentrate, dry, pulverize, and pass through a 120-mesh sieve; fillers, disintegrants, auxiliary The suspension was sieved through a 100-mesh sieve and set aside. Weigh and mix genistein inclusion compound, sucrose, mannitol, hydroxypropyl starch and xanthan gum according to the prescription amount, stir for more than 30 minutes, and mix evenly. Stir and add 10% polyvinylpyrrolidone ethanol solution into the above-mentioned mixed main and auxiliary materials to make a soft material. Granulate with 20-mesh sieve, dry at 55-60°C; granulate with 18-mesh sieve. Packed in composite film bags or bottles.
Embodiment 3
[0030] Embodiment 3: the preparation of genistein dropping pill
[0031] Take 100g of polyethylene glycol 4000 according to the prescription, heat and melt it in a water bath at 80°C, add 50g of genistein under stirring, stir at 80°C to make it disperse evenly, and transfer the liquid medicine to the dropping pill machine for constant temperature storage In the material tank, the dropping speed is 60 drops / min, the coolant is liquid paraffin, and the temperature of the coolant is 15° C., the weight of the dropping pills is adjusted to 60 mg, and the dropping pills are made, and the surface coolant of the dropping pills is removed, and then subpackaged to obtain final product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com