Polysubstituted nitrogen-containing heteroaromatic compound, and preparation method and application thereof
A technology for aromatic heterocycles and compounds, applied in the field of multi-substituted nitrogen-containing aromatic heterocycles and their preparation, can solve the problems of insufficient fluorescence properties of compounds, inability to construct pyridinamine at one time, poor atom economy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
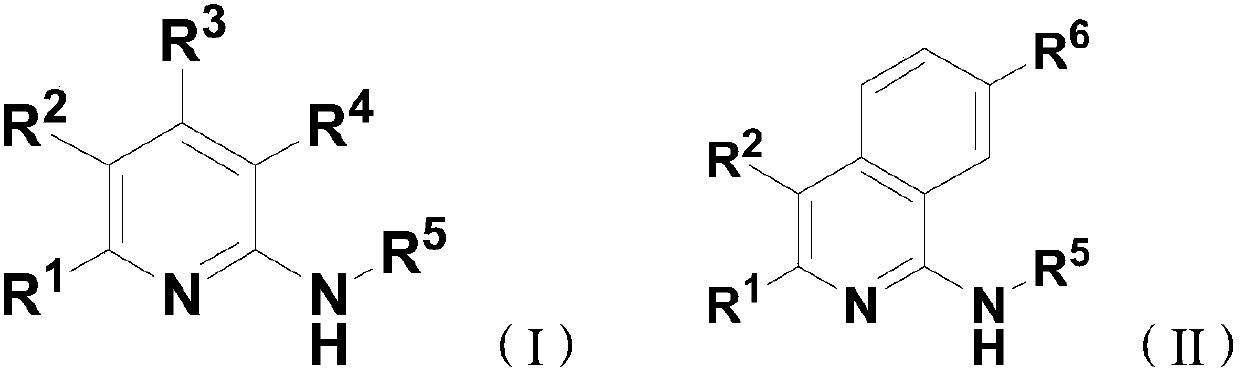
[0050] This embodiment provides a new compound 2-tert-butylamino-6-phenylpyridine-3,4-dicarboxylic acid ethyl ester, its molecular formula is: C 21 h 26 N 2 o 4 , whose structure is:
[0051]
[0052] The compound is synthesized through the following specific steps:
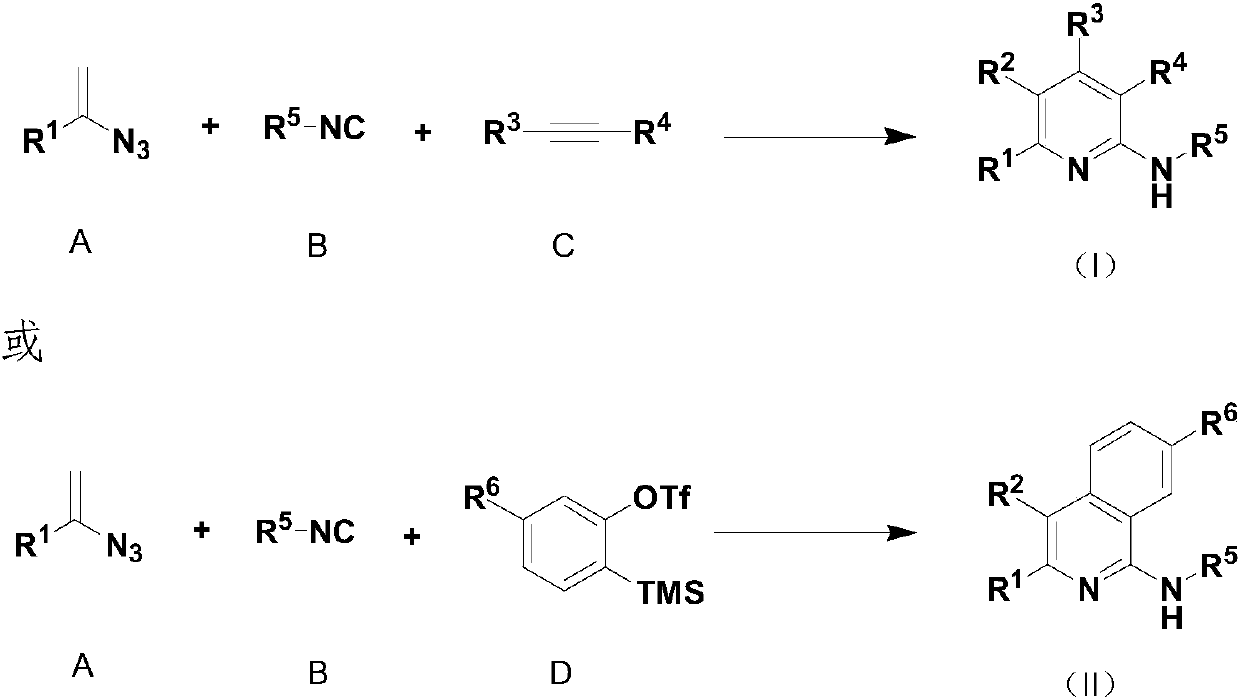
[0053] Add {Rh(COD)Cl}2 (2mg, 0.0038mmol) and 2,2'-bipyridine (1mg, 0.0075mmol), 1,4-dioxane (2ml) in sequence to a 5ml reaction tube, and stir for 5min Afterwards, (1-azidovinyl)benzene (22mg, 0.15mmol) and tert-butylisonitrile (13mg, 17uL, 0.2mmol) were added using a syringe, and reacted at room temperature for 5h. After the reaction was complete, diethyl butynoate (51mg, 48uL, 0.3mmol) was added with a syringe, heated to 120°C for 5h, and TLC detected the complete reaction. The solvent was concentrated under reduced pressure, and the residue was separated and purified by flash column chromatography (petroleum ether: ethyl acetate = 50:1) to obtain 40 mg of a yellow-green solid with a yield of 71%.
[...
Embodiment 2
[0061] Prepare brand-new compound C according to the method described in Example 1 22 h 28 N 2 o 4, the product yield is 60%; the compound structural formula is:
[0062]
[0063] Characterization data for the obtained compounds include:
[0064] 1 H NMR (400MHz, CDCl 3 )δ7.99–7.91(m,3H),7.32–7.21(m,2H),6.94(s,1H),4.42–4.23(m,4H),2.41(s,3H),1.56(s,9H) ,1.42–1.30(m,6H).
[0065] 13 C NMR(101MHz,cdcl3)δ169.17,167.10,159.30,157.88,145.90,140.25,135.79,129.51,127.32,105.53,100.73,61.80,61.36,51.82,29.32,21.52,144.167
[0066] IR (neat) 2954.64, 1737.67, 1686.74, 1558.48, 1369.89, 1289.68, 1182.11, 1044.46.
[0067] HRMS(ESI+)calcd for C 22 h 29 N 2 o 4 :385.2127,found:385.2108.
Embodiment 3
[0069] Prepare brand-new compound C according to the method described in Example 1 22 h 28 N 2 o 4 , the product yield is 68%; the compound structural formula is:
[0070]
[0071] Characterization data for the obtained compounds include:
[0072] 1 H NMR (400MHz, CDCl 3 )δ7.97(s,1H),7.89–7.83(m,2H),7.39–7.33(m,1H),7.25(d,J=6.4Hz,1H),6.96(s,1H),4.36(q ,J=7.2Hz,2H),4.30(q,J=7.2Hz,2H),2.43(s,3H),1.56(s,9H),1.38(t,J=7.2Hz,3H),1.34(t ,3H).
[0073] 13 C NMR (75MHz, CDCl3) δ169.12, 167.09, 159.45, 157.85, 145.89, 138.52, 138.28, 130.77, 128.68, 128.05, 124.59, 105.88, 101.00, 61.80, 61.39, 51.812, 24.14, 29.3
[0074] IR (neat) 2917.53, 1737.76, 1686.83, 1559.92, 1369.89, 1255.88, 1229.12, 1178.37.
[0075] HRMS(ESI+)calcd for C 22 h 29 N 2 o 4 :385.2127,found:385.2112.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com