Self-healing photoresponsive supramolecular fluorescent hydrogel and preparation method and application thereof
A supramolecular, light-responsive technology, applied in the field of supramolecular chemistry, can solve the problems of not meeting the needs of the society, less light-responsive fluorescent hydrogels, etc., achieving low production cost, good self-healing, and excellent self-healing performance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
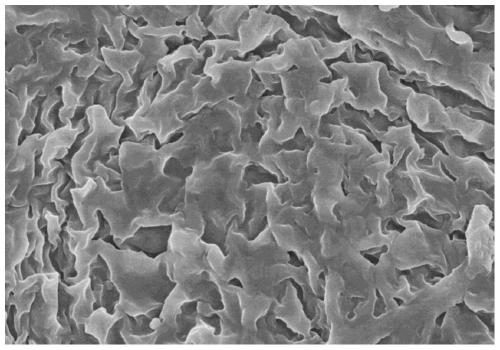
Image
Examples
preparation example Construction
[0048] A preparation method of self-healing photoresponsive supramolecular fluorescent hydrogel, comprising the following steps:
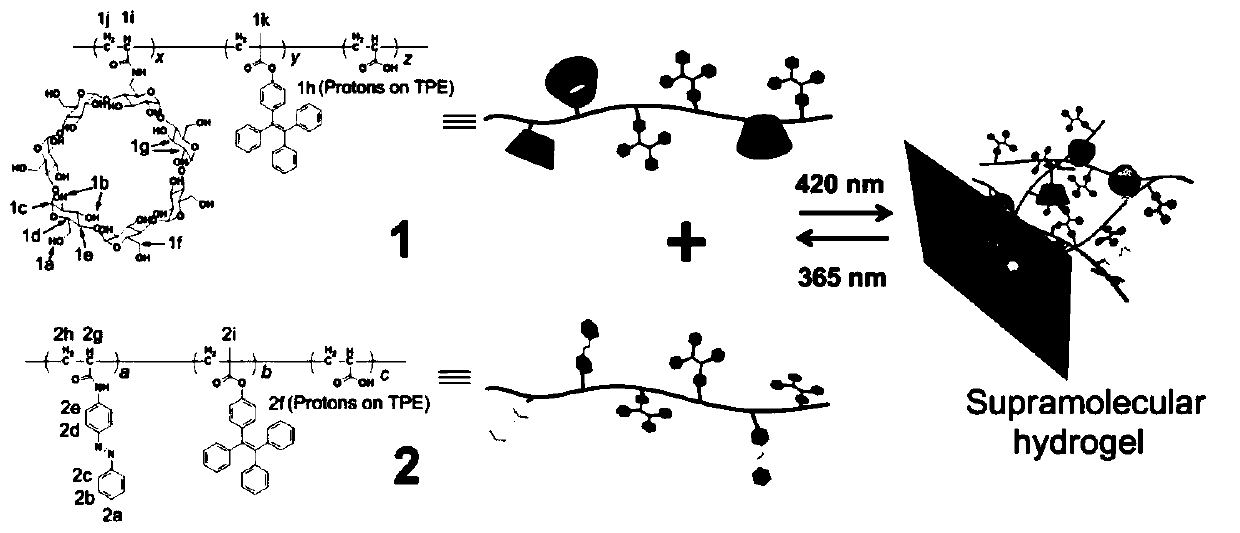
[0049] (A) Preparation of Polymer 1: Dissolve β-cyclodextrin monomer, tetraphenylethylene monomer, and acrylic acid in dimethyl sulfoxide, stir the resulting mixture for the first time, bubble and add azobisisobutyronitrile , rapid cooling and quenching after secondary stirring, the obtained polymer solution was dropped into diethyl ether, the precipitated solid was collected by repeated filtration, and the obtained solid was dried to obtain polymer 1;
[0050] (B) Preparation of Polymer 2: Dissolve azo compound monomer, tetraphenylethylene monomer, and acrylic acid in benzene, stir and bubble the resulting mixture for the first time and add azobisisobutyronitrile, and speed up after the second stirring. Cooling quenched, the resulting polymer solution was dropped into diethyl ether, the precipitated solid was collected by filtration several times,...
Embodiment 1
[0058] Preparation of Polymer 1:
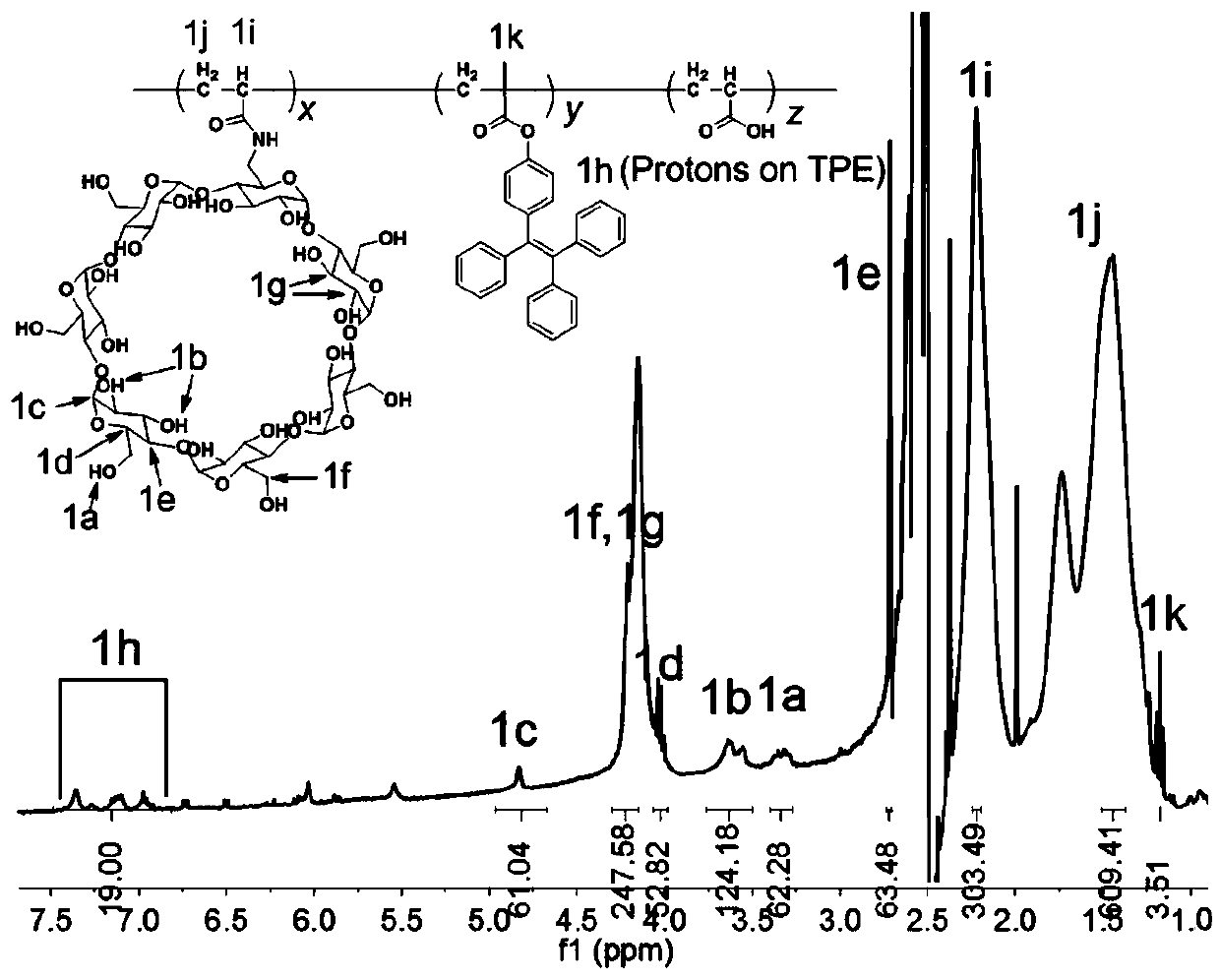
[0059] Polymer 1 was prepared by radical polymerization from β-cyclodextrin 4, tetraphenylethylene 5 and acrylic acid. Specific operation: β-cyclodextrin monomer 4, tetraphenylethylene monomer 5 and acrylic acid were dissolved in dimethyl sulfoxide (DMSO), and the resulting mixture was stirred at room temperature and bubbled with argon (Ar) for 30 minute. Azobisisobutyronitrile was added in one portion and the mixture was stirred at 70°C for 24 hours, then the polymerization was quenched by liquid nitrogen snap freezing. The resulting mixed solution was dropped into ether, and the precipitated solid was collected by vacuum filtration. After repeated filtration three times, the collected polymer was vacuum-dried to obtain a total of 12.47 g of polymer 1 with a yield of 55.4%. The structure of polymer 1 is shown in the following formula:
[0060]
[0061] polymer 1 about 1 The data of H-NMR, Mn, Mw, and PDI are shown in the following tab...
Embodiment 2
[0064] Preparation of Polymer 2:
[0065] Polymer 2 was prepared from azo compound 6, tetraphenylethylene 5 and acrylic acid by radical polymerization. Azo compound monomer 6, tetraphenylethylene monomer 5 and styrene were dissolved in benzene, and the resulting mixture was stirred at room temperature and bubbled with argon (Ar) for 30 minutes. Azobisisobutyronitrile was added in one portion and the mixture was stirred at 70°C for 24 hours, then the polymerization reaction was quenched by liquid nitrogen flash freezing. The resulting mixture was dropped into diethyl ether and the precipitated solid was collected by vacuum filtration. After repeated filtration three times, the collected polymer was vacuum-dried to obtain a total of 22.05 g of polymer 2 with a yield of 58.1%. The structure of polymer 2 is shown in the following formula:
[0066]
[0067] polymer 2 about 1 The data of H-NMR, Mn, Mw, and PDI are shown in the following table:
[0068]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com