Synthesis process for promestriene
A synthesis process, a technology for proestrene, is applied in the field of synthesis of proestrene, can solve problems such as being unsuitable for industrial production, large safety risks, etc., and achieves avoiding the use of highly toxic raw materials, reducing production costs, reducing pollution and safety. hidden effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
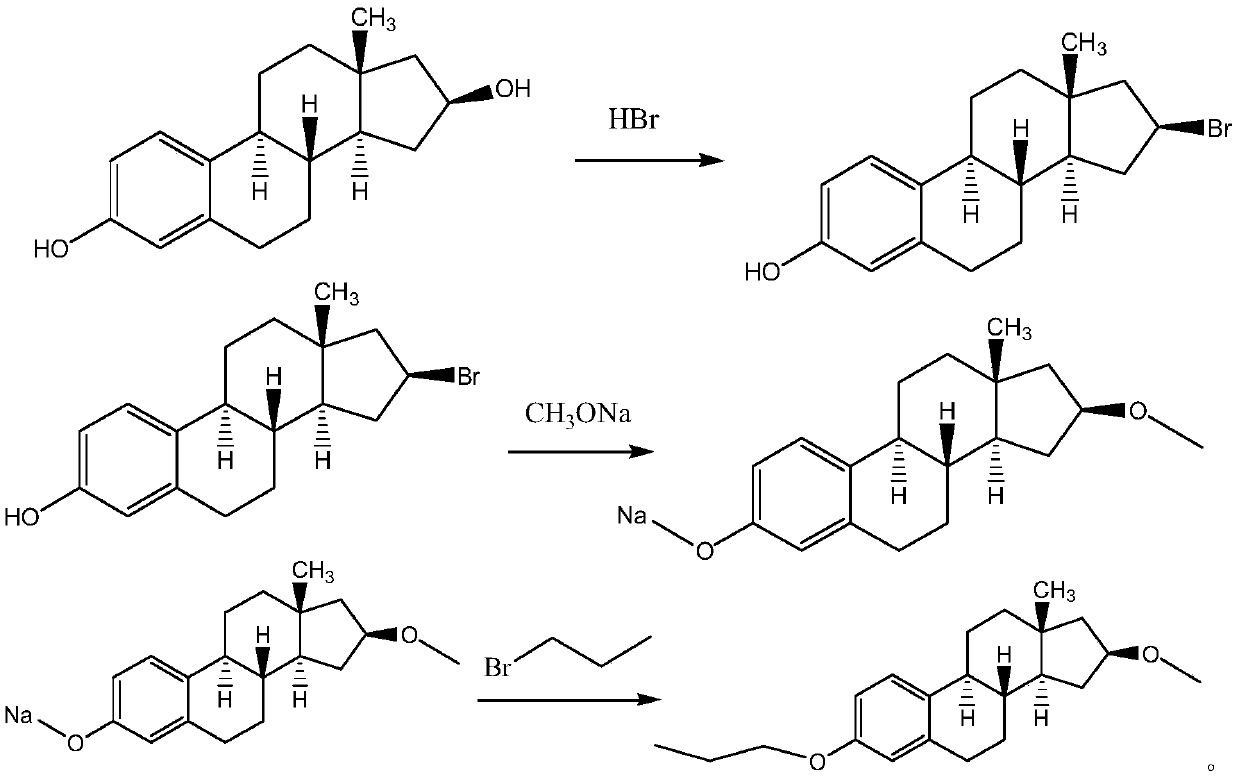
[0037] Add 10g of estradiol and 200g of acetone to a 500ml four-necked bottle under the protection of nitrogen at room temperature, stir to dissolve, add 10g of sulfonated carbon-based solid acid catalyst, add 18.97g of 47wt.% hydrobromic acid dropwise at a temperature of 25±5°C, and drop Raise the temperature to 55°C and stir the reaction for 24 hours. After the reaction, filter and recover the solid acid catalyst, add 200g water to the filtrate and stir for 0.5 hour, extract 3 times with 200g ethyl acetate, combine the organic phases and wash 2 times with water, dry over anhydrous magnesium sulfate, and concentrate to After drying, an off-white solid was obtained, which was recrystallized from methanol and dried by filtration to obtain 11.08 g of a white solid. That is, 3-hydroxy-17β-bromoestro-1,3,5,(10)-triene, with a yield of 90% and a purity of 99.2% by HPLC.
[0038] Add 11.08g of 3-hydroxy-17β-bromoestro-1,3,5,(10)-triene to a 500ml four-neck flask under nitrogen prote...
Embodiment 2
[0040] Add 20g of estradiol and 400g of acetone to a 1000ml four-neck bottle under the protection of nitrogen at room temperature, stir and dissolve, add 20g of ZSM-5 molecular sieve catalyst, add 25.29g of 47wt.% hydrobromic acid dropwise at a temperature of 25±5°C, and raise the temperature to Stir and react at 55°C for 24 hours. After the reaction, filter and recover the solid acid catalyst, add 400g water to the filtrate and stir for 0.5 hour, extract 3 times with 400g ethyl acetate, combine the organic phases and wash twice with water, dry over anhydrous magnesium sulfate, and concentrate to dryness. An off-white solid was obtained, which was recrystallized from methanol, filtered and dried to obtain 22.41 g of a white solid. That is, 3-hydroxy-17β-bromoestro-1,3,5,(10)-triene, with a yield of 91% and a purity of 99.4% by HPLC.
[0041]Add 22.41g of 3-hydroxy-17β-bromoestro-1,3,5,(10)-triene to a 1000ml four-neck flask under the protection of nitrogen at room temperature,...
Embodiment 3
[0043] Add 30g of estradiol and 600g of acetone to a 1000ml four-necked flask at room temperature under nitrogen protection, stir to dissolve, add 30g of sulfonated carbon-based solid acid catalyst, add 44.26g of 47wt.% hydrobromic acid dropwise at a temperature of 25±5°C, and drop Raise the temperature to 55°C and stir the reaction for 24 hours. After the reaction, filter and recover the solid acid catalyst, add 600 g of water to the filtrate and stir for 0.5 hours, extract 3 times with 600 g of ethyl acetate, combine the organic phases and wash with water twice, dry over anhydrous magnesium sulfate, and concentrate to After drying, an off-white solid was obtained, which was recrystallized from methanol and dried by filtration to obtain 33.49 g of a white solid. That is, 3-hydroxy-17β-bromoestro-1,3,5,(10)-triene, with a yield of 91% and a purity of 99.6% by HPLC.
[0044] Add 33.49g of 3-hydroxy-17β-bromoestro-1,3,5,(10)-triene to a 1000ml four-neck flask under the protectio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com