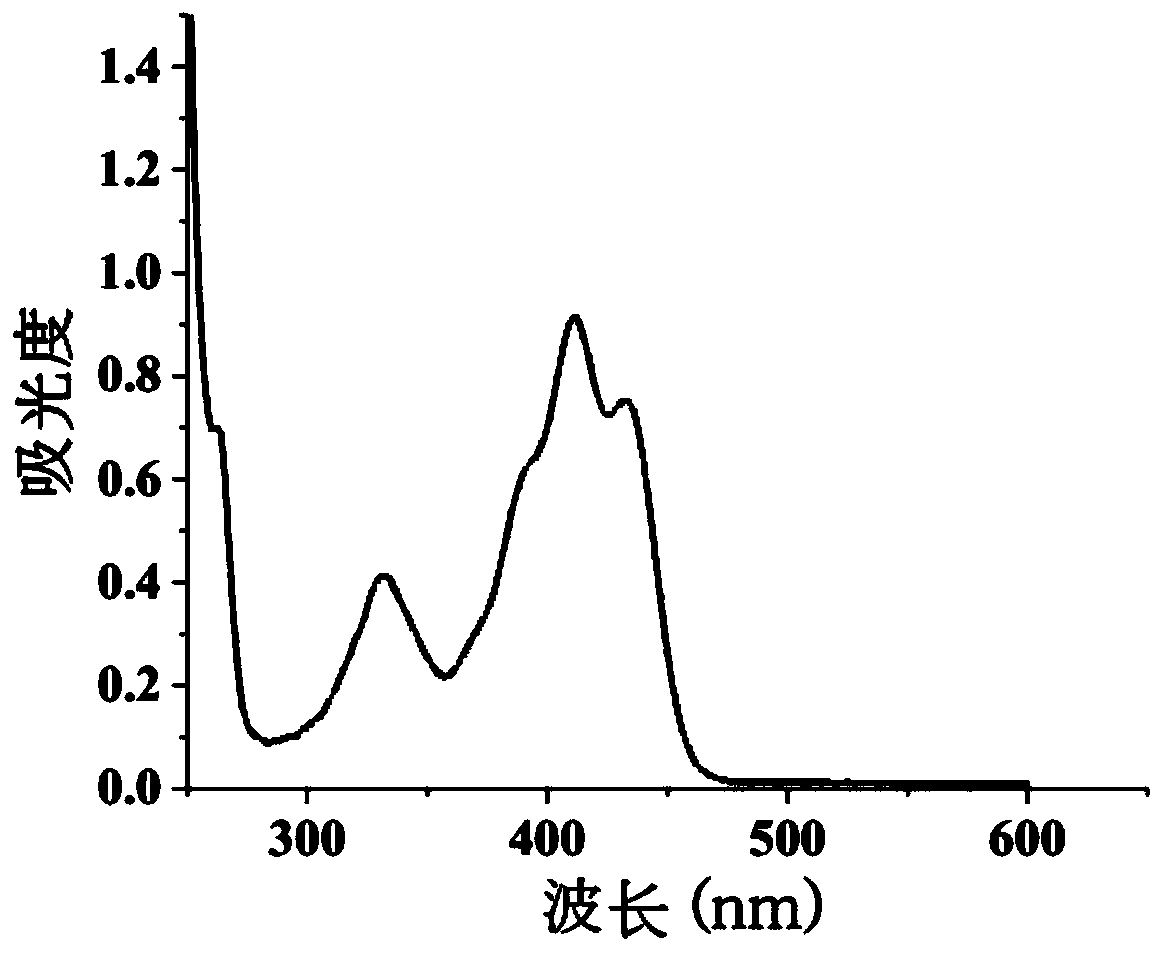
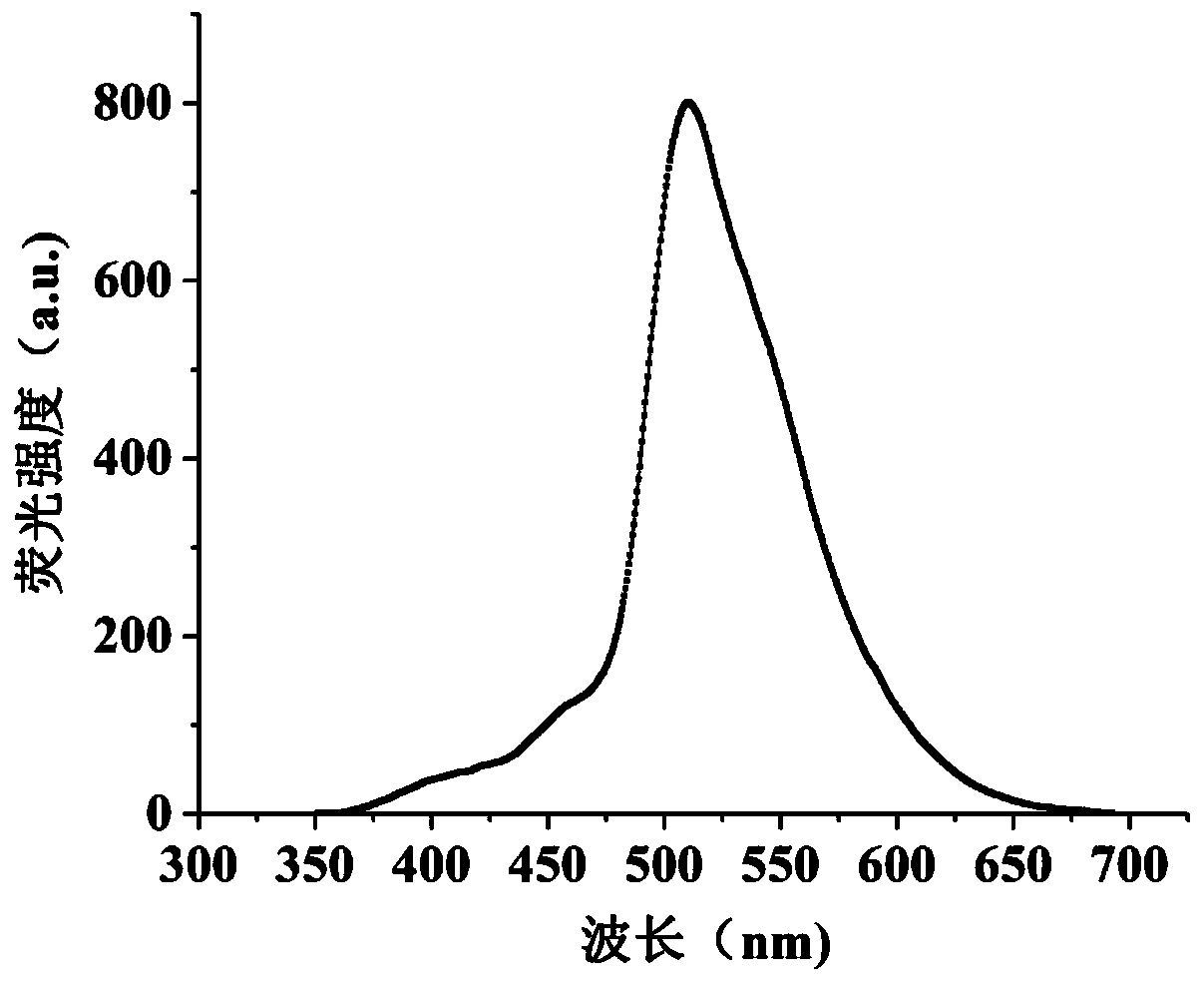
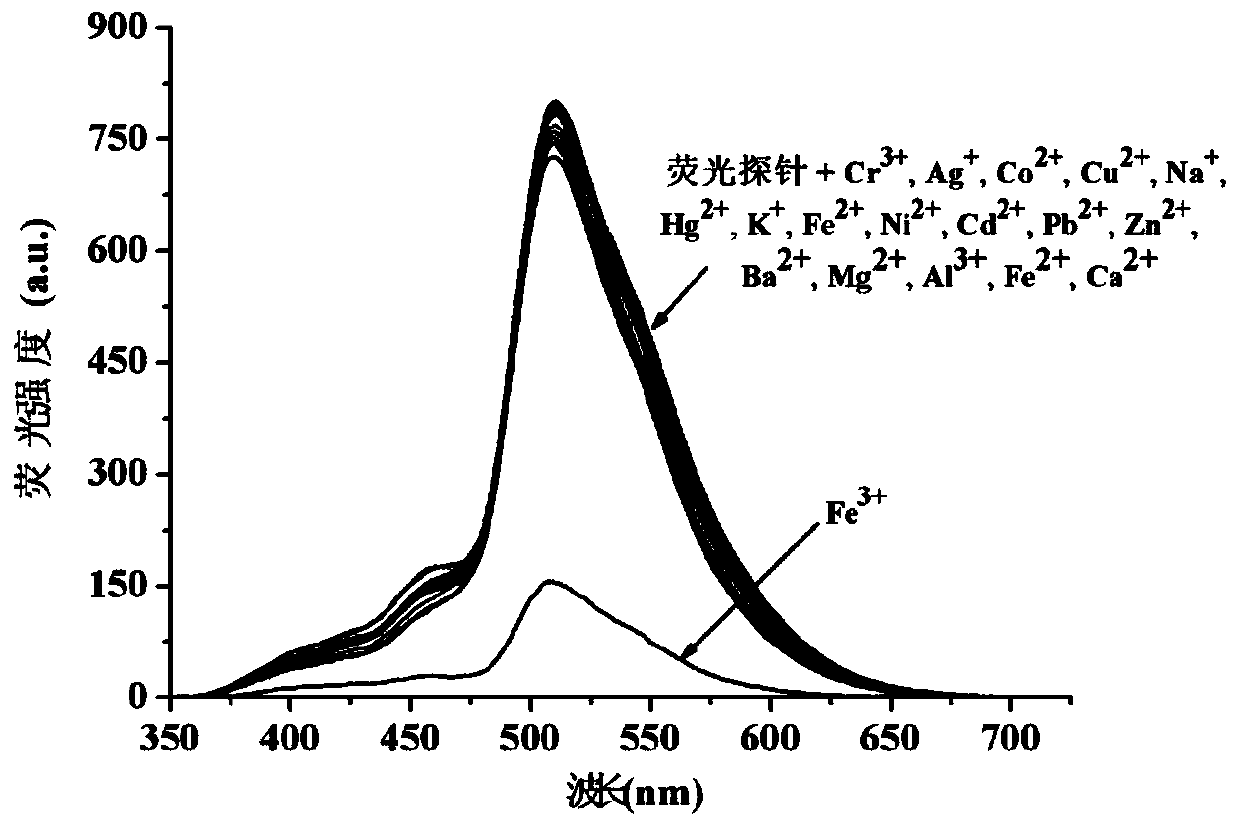
Synthesis method and application method of benzothiazole di-Schiff base fluorescent molecular probe for detection of iron ions
A technology of benzothiazole double Schiff base and fluorescent molecular probe, which is applied in the fields of fluorescence/phosphorescence, chemical instruments and methods, and material analysis through optical means, and can solve poor selectivity and sensitivity, and poor water solubility of fluorescent probes , easy to be affected by pH and other problems, to achieve the effect of simple steps, strong anti-interference ability, and simple and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] The synthetic method specifically comprises the following steps:
[0036] 1) Add benzothiazole-2-carbaldehyde to 2-hydroxy-1-naphthalene hydrazine in a molar ratio of 1:(1~2) into an acidic solvent, heat under reflux to 75-80°C, react for 5~7 hours, and pump while it is hot Filter to obtain the reaction mixture;
[0037] 2) Recrystallize the reaction mixture using a mixed solvent of acetone and water, and precipitate a large amount of solid after cooling; filter and collect the filter cake to obtain a benzothiazole bis-Schiff base fluorescent molecular probe (BP) for iron ion detection.
[0038] Wherein, the acidic solvent described in step 1) is glacial acetic acid, or ethanol solvent of concentrated sulfuric acid or concentrated hydrochloric acid, and the concentration of concentrated sulfuric acid or concentrated hydrochloric acid in the ethanol solvent of concentrated sulfuric acid or concentrated hydrochloric acid is 5%-10%. The volume ratio of acetone and water i...
Embodiment 1
[0044] Synthetic method of benzothiazole double Schiff base fluorescent molecular probe for iron ion detection:
[0045] 1) Add 163 mg (1 mmol) of benzothiazole-2-carbaldehyde and 172 mg (1 mmol) of 2-hydroxy-1-naphthylhydrazine into 15 mL of glacial acetic acid, reflux for 5 to 7 hours, and suction filter while hot to obtain a reaction mixture;
[0046] 2) Recrystallize the filter cake with acetone and water to obtain a fluorescent molecular probe, which is a benzothiazole double Schiff base ratio fluorescent molecular probe for iron ion detection. The calculated yield was 71%.
[0047] The mass spectrometry and elemental analysis of the benzothiazole double-Schiff base ratio fluorescent molecular probe used for the detection of iron ions prepared in this example and its detection in deuterium-substituted dimethyl sulfoxide DMSO solvent 1 H NMR spectral data are as follows:
[0048] 1 H NMR(500MHz,DMSO)δ12.18(s,1H),8.88(s,1H),8.64(s,1H),7.88(t,J=9.3Hz,2H),7.76(d,J=7.4Hz ,...
Embodiment 2
[0061] The preparation method of the benzothiazole bis-Schiff base ratio fluorescent molecular probe for iron ion detection is as follows:
[0062] 1. According to the molar ratio of benzothiazole-2-formaldehyde and 2-hydroxyl-1-naphthalene hydrazine as 1:(1~2), add benzothiazole-2-formaldehyde and 2-hydroxyl-1-naphthalene hydrazine to In the ethanol solvent of concentrated sulfuric acid, reflux for 5 to 7 hours, and suction filter while it is hot to obtain the reaction mixture;
[0063] 2. Recrystallize the reaction mixture with a mixed solvent of acetone and water, and precipitate a large amount of solids after cooling; filter and collect the filter cake to obtain the fluorescent molecular probe BP, which is the ratio of benzothiazole bis-Schiff base for iron ion detection Fluorescent Molecular Probes. The calculated yield was 74%.
[0064] The mass spectrometry and elemental analysis of the benzothiazole double Schiff base ratio fluorescent molecular probe for the detecti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com