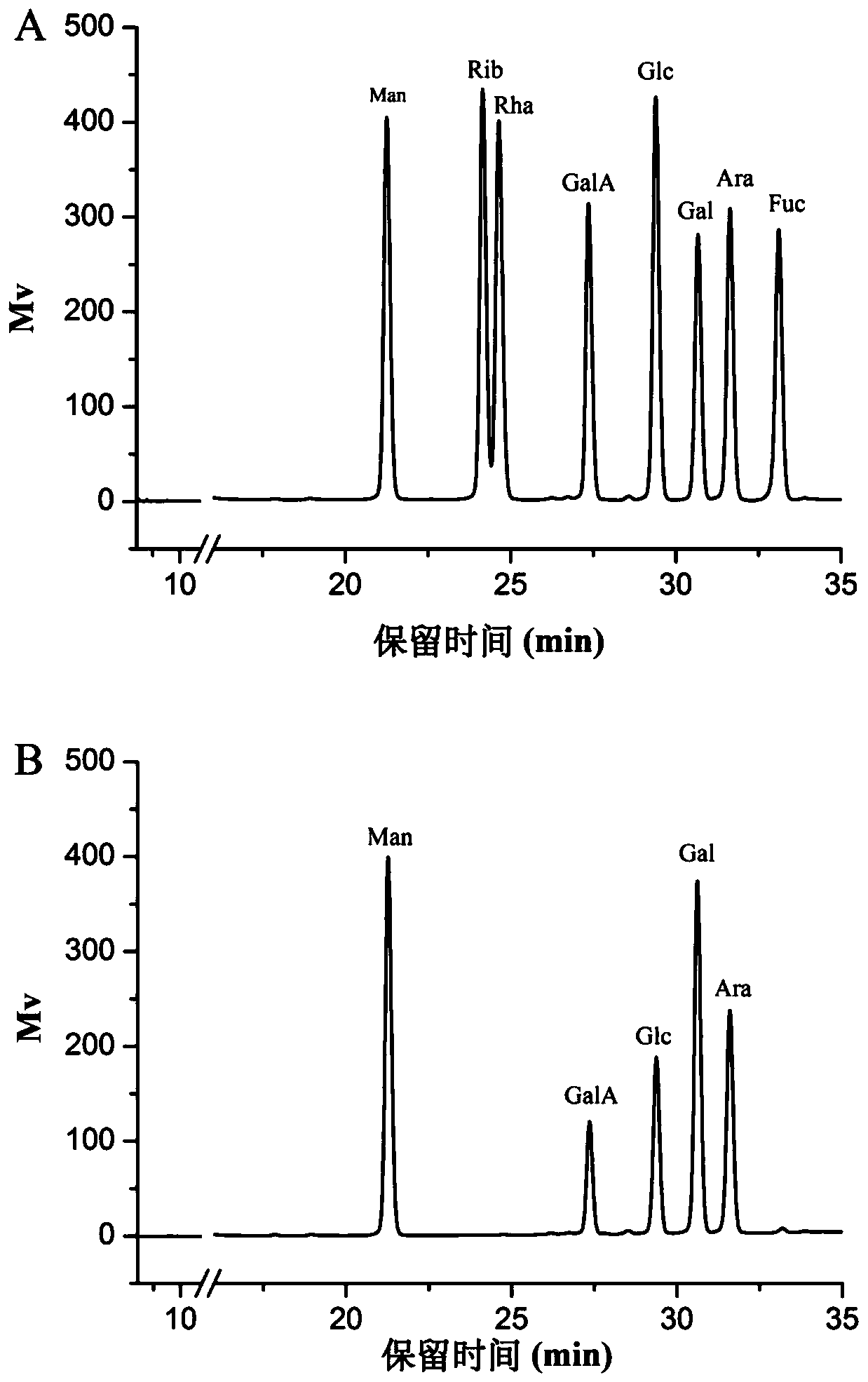
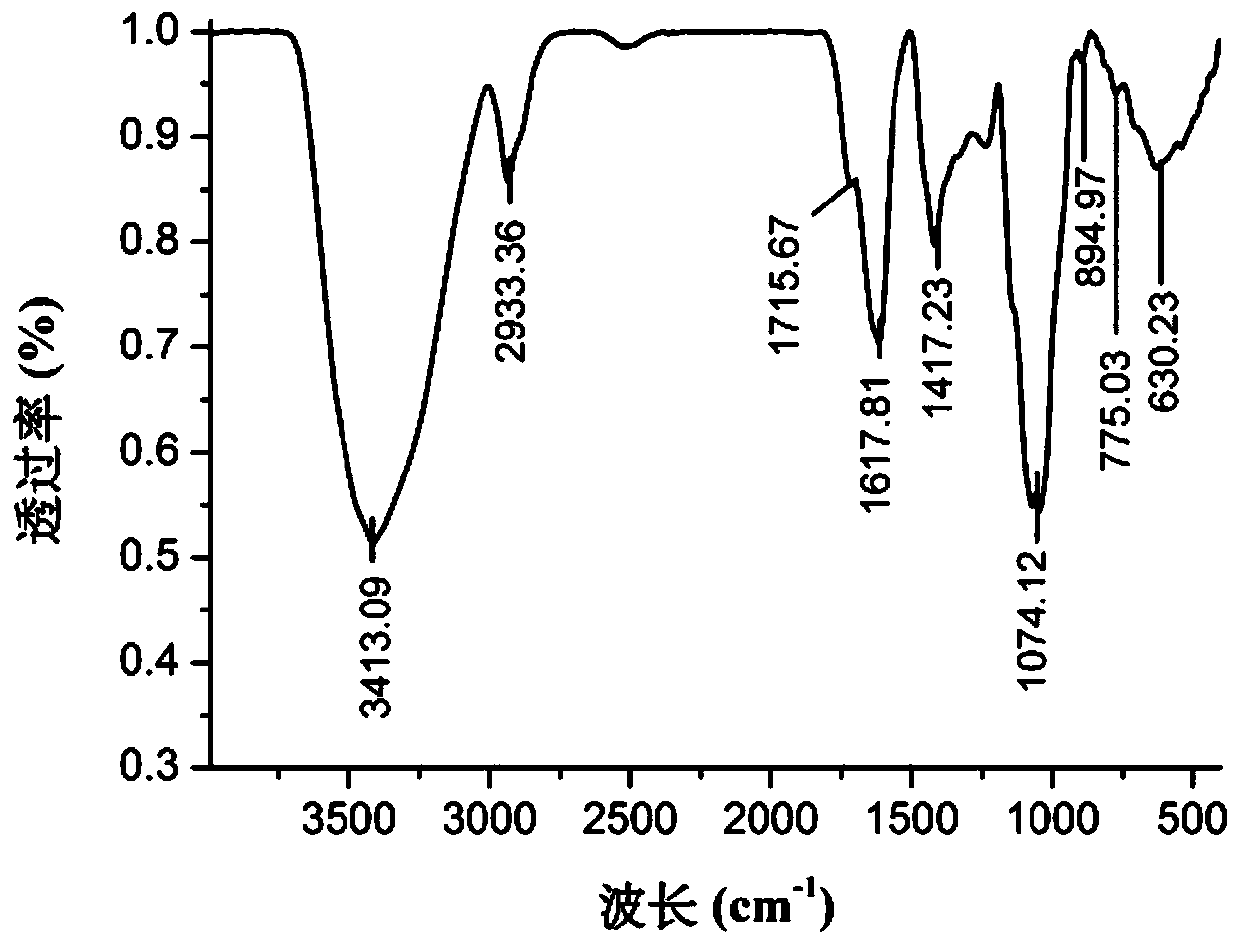
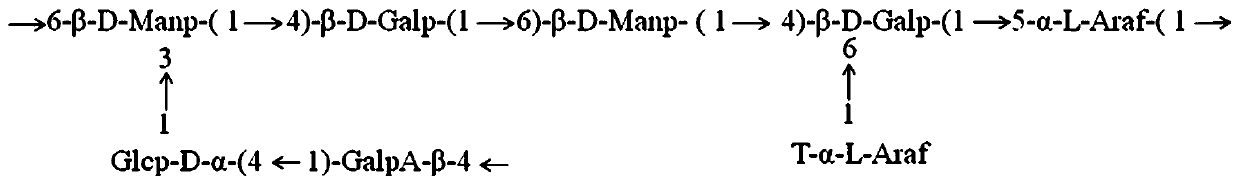Mallotus furetianus homopolysaccharide as well as preparation method and application thereof
A technology of camellia kucha and polysaccharides, applied in the field of natural polymers, to achieve the effects of high-efficiency DPPH free radicals, anti-oxidation and inhibition of α-glucosidase activity, and scavenging DPPH free radicals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0041] Implementation case 1: Preparation of Camellia polysaccharide MFP-2A
[0042] (1) Polysaccharide extraction:
[0043] Take 1000g of the washed and dried camellia, pulverize it with a pulverizer, and pass it through a 60-mesh sieve. Add 6L of absolute ethanol and reflux for two hours in a water bath at 70℃. Dry in a blast oven, add 20L of water, extract for 4 hours at 80°C in a water bath, centrifuge, filter, concentrate to 2.5L, and slowly add four times the volume of 10L absolute ethanol at 500r / min. Let it stand for 12 hours at -4°C, centrifuge, collect the precipitate, and then wash it with absolute ethanol and acetone three times in sequence. The precipitate is freeze-dried to obtain 30.4 g crude polysaccharide of camellia.
[0044] (2) In addition to protein:
[0045] Dissolve 20 g of the above-prepared camellia crude polysaccharide in 300 mL of water, stir, centrifuge, add Sevag reagent to the supernatant, shake, stand still, centrifuge, take the supernatant, then add Se...
Embodiment example 2
[0060] Implementation case 2: Antioxidant activity experiment of camellia polysaccharide MFP-2A
[0061] The anti-oxidant activity of polysaccharides was evaluated by DPPH, ABTS method and superoxide radical experiment, as follows:
[0062] Scavenging DPPH free radicals: Put 150μL of MFP-2A solution (0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.4, 1.6, 1.8 and 2.0mg / mL) and 150μL of DPPH solution (60μmol / L) in a 96-well plate respectively Mix and react for 30 minutes in the dark, test the absorbance of the mixture at 517nm, and use ascorbic acid (Vc) as a positive control. Figure 5 A.
[0063] Scavenging ABTS free radicals: Mix 2 mL of ABTS (7 mmol / L) solution and 2 mL of potassium persulfate aqueous solution (2.45 mmol / L), and react for 12 hours in the dark to prepare cationic ABTS free radicals. The prepared cationic ABTS radical solution was diluted with ethanol to an absorbance value of 0.7±0.05 at 734nm. Add 20μL of polysaccharide solution (0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.4, 1.6, 1.8 and ...
Embodiment example 3
[0066] Implementation case 3: Camellia polysaccharide MFP-2A inhibits α-glycosidase activity experiment
[0067] Add 20μL of polysaccharide solution (0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.4, 1.6, 1.8 and 2.0mg / mL) and 40μL of α-glycosidase solution (0.2U / mL) to a 96-well plate, mix well, Incubate for 10 min at 37℃, then add 20.0μL pNPG (10mM), mix well, react at 37℃ for 30min, and finally add 100μL Na 2 CO 3 (0.2M) solution to stop the reaction, test the absorbance of the mixture at 405nm, take acarbose as a positive control, see the result Image 6 .
[0068] The results showed that Camellia polysaccharide MFP-2A has inhibitory α-glycosidase activity in the concentration range of 0.2-2.0 mg / mL, and its inhibitory activity increases with the increase of concentration. At a concentration of 2.0 mg / mL, the inhibition rate of MFP-2A was 68.42±1.21%. It shows that Camellia polysaccharide has a good inhibitory effect on α-glycosidase activity.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com