Preparation method of 4-bromine-3-methyl-2-butene-1-alcohol acetate
A technology of alcohol acetate and methyl, applied in the field of preparation of 4-bromo-3-methyl-2-buten-1-ol acetate, can solve low product yield and excess isoprene Many problems, low safety operation coefficient, etc., to achieve the effect of simple post-treatment process, increased reaction rate, and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
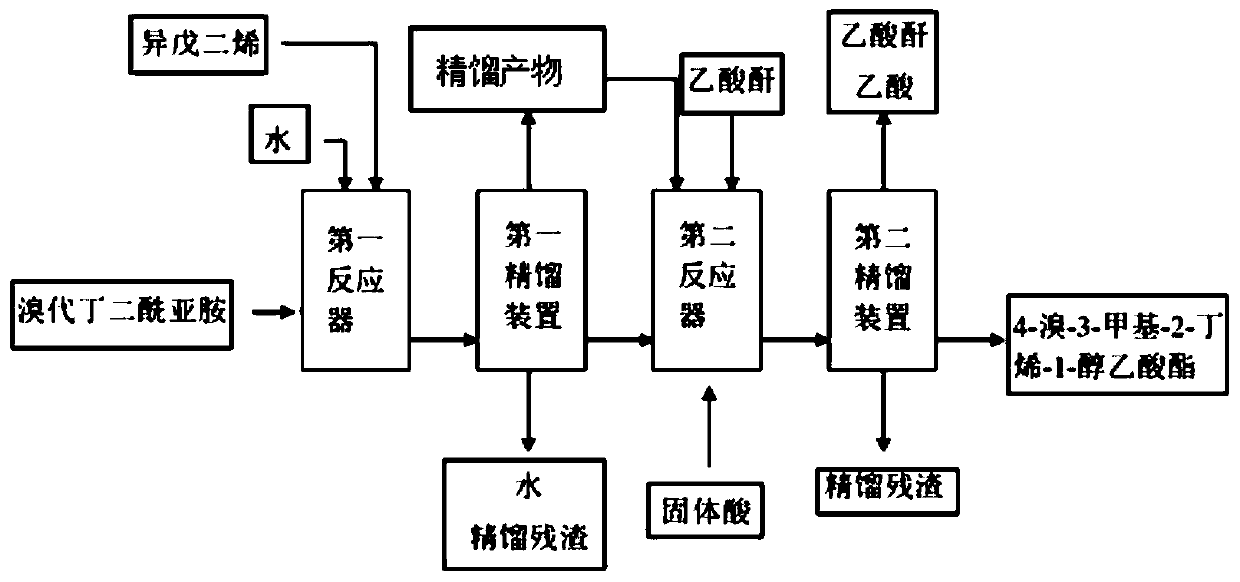
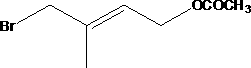
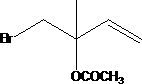
[0041] 187g of bromosuccinimide solid particles were charged into the tube reactor, with a volume of about 100ml. Weigh 68g of isoprene and 20g of water, start the metering pump to continuously pump the materials in, the flow rate of the pump is 40ml / h, and control the reaction temperature to 0°C. The reaction is over, and the gas phase detection isoprene reaction is complete, and rectification in a rectification tower obtains 110.8g of rectification products (4-bromo-3-methyl-2-butene-1-alcohol and 1-bromo-2-methyl -3-buten-2-ol), the yield was 92%. After mixing 110.8g of rectified products and 100g of acetic anhydride, pump them into a pipeline reactor filled with 100g of D001 type strongly acidic cation exchange resin, the pump flow rate is 50ml / h, and the esterification rearrangement reaction temperature is 100°C. At the end of the reaction, there is no 1,2-addition isomer in the gas phase detection. First, the acetic acid and unreacted acetic anhydride generated by the r...
Embodiment 2
[0043] 190 g of bromosuccinimide solid particles are charged into the tube reactor, with a volume of about 100 ml. Weigh 68g of isoprene and 25g of water, start the metering pump to continuously pump the materials in, the flow rate of the pump is 50ml / h, and control the reaction temperature to 5°C. The reaction is over, and the gas phase detection isoprene reaction is complete, and rectification in a rectification tower obtains 108.5g rectification products (4-bromo-3-methyl-2-butene-1-alcohol and 1-bromo-2-methyl -3-buten-2-ol), the yield is 90%. After mixing 108.5g of rectified product and 105g of acetic anhydride, pump them into a pipeline reactor filled with 100g of 732 type strong acid cation exchange resin, the pump flow rate is 20ml / h, and the esterification rearrangement reaction temperature is 80°C. At the end of the reaction, there is no 1,2-addition isomer in the gas phase detection. First, the acetic acid and unreacted acetic anhydride generated by the reaction ar...
Embodiment 3
[0045] 187g of bromosuccinimide solid particles were charged into the tube reactor, with a volume of about 100ml. Weigh 68g of isoprene and 20g of water, start the metering pump to continuously pump the materials in, the flow rate of the pump is 20ml / h, and control the reaction temperature to 10°C. The reaction is over, and the gas phase detection isoprene reaction is complete, and rectification in a rectification tower obtains 113g rectification products (4-bromo-3-methyl-2-butene-1-alcohol and 1-bromo-2-methyl- 3-buten-2-ol), yield 93.8%. After mixing 113g of rectified products and 110g of acetic anhydride, pump them into a pipeline reactor filled with 100g of 732 type strongly acidic cation exchange resin, the pump flow rate is 40ml / h, and the esterification rearrangement reaction temperature is 90°C. At the end of the reaction, there is no 1,2-addition isomer in the gas phase detection. First, the acetic acid and unreacted acetic anhydride generated by the reaction are re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com