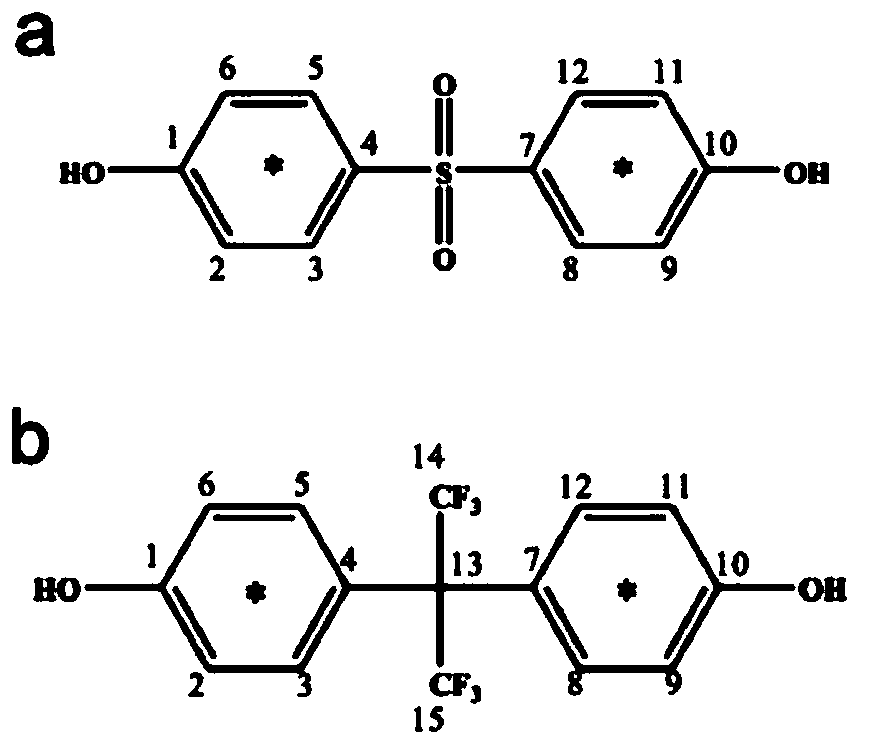
Preparation method of carbon isotope-labeled bisphenols and bisphenols AF
A carbon isotope and double-labeled technology, which is applied in the synthesis of carbon isotope-labeled compounds, can solve the problems of low yield of bisphenol AF, and achieve the effect of simple preparation process, avoiding complicated purification steps and simple synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The present invention provides a preparation method of carbon isotope-labeled bisphenol S, and the carbon isotope-labeled bisphenol S comprises: 13 C-labeled bisphenol S or 14 C-labeled bisphenol S, including the following steps:
[0031] The carbon isotope-labeled phenol is mixed with sulfuric acid, and the sulfonation reaction and the condensation to sulfone reaction are carried out successively in a protective atmosphere, and then the obtained condensation-to-sulfone product system is separated and purified by normal phase preparative liquid chromatography or normal phase preparative thin-layer chromatography. Obtain carbon isotope-labeled bisphenol S; the carbon isotope-labeled phenol includes 13 C-labeled phenol or 14 C-labeled phenol.
[0032] In the preparation method of carbon isotope-labeled bisphenol S provided by the present invention, when the 13 When C-labeled phenol is a raw material, the prepared product is 13 C-labeled bisphenol S; 14 When C-labele...
Embodiment 1
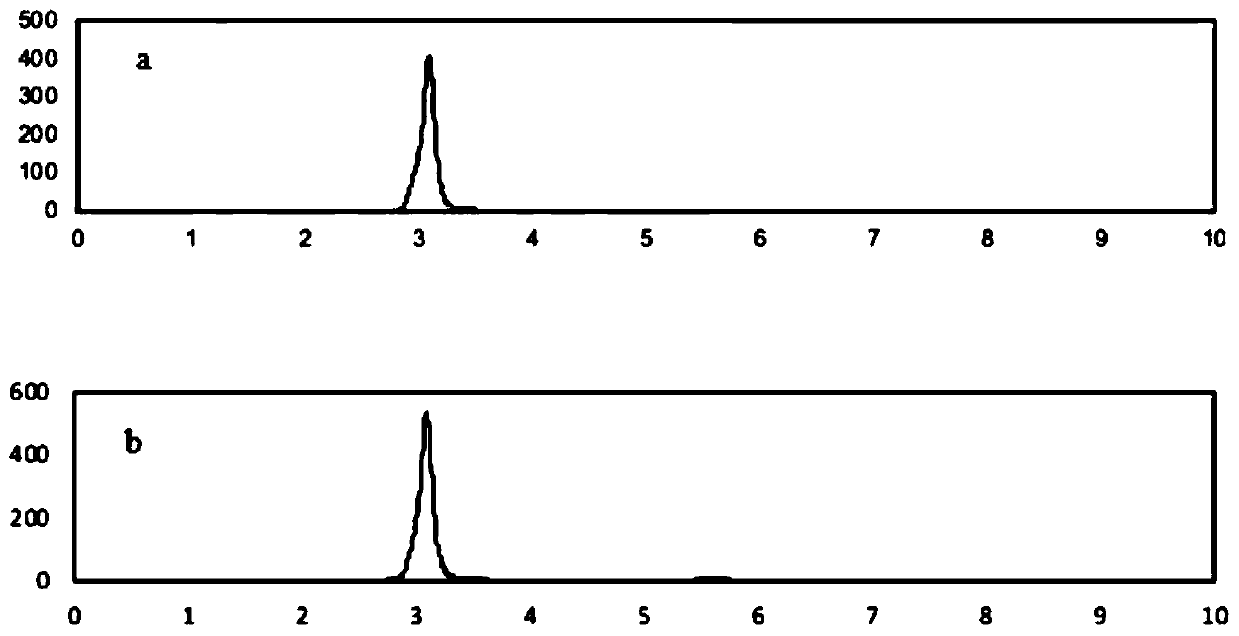
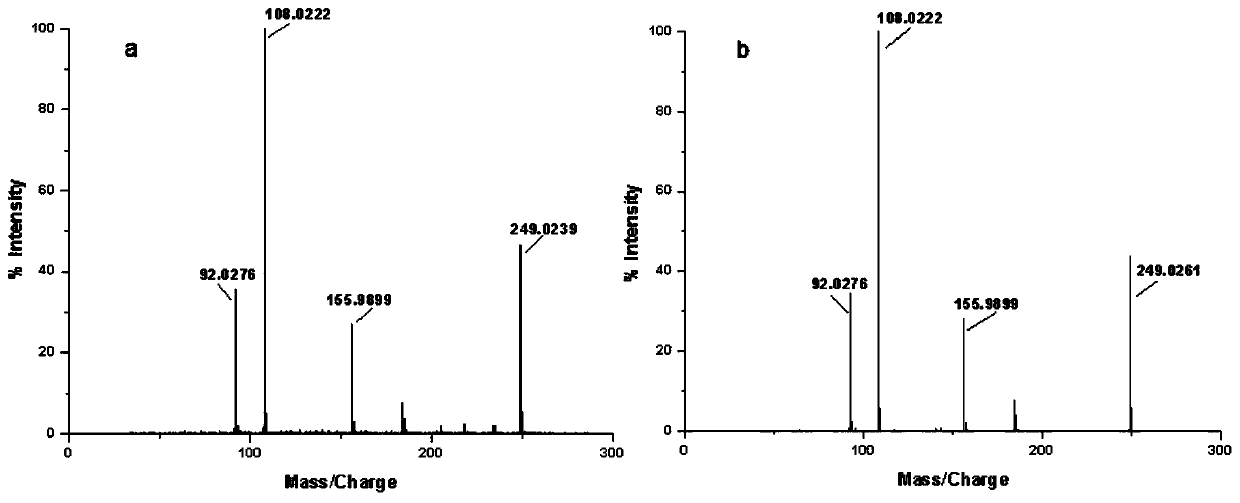
[0064] First, unlabeled bisphenol S was prepared to determine the reaction conditions, and the synthesized unlabeled bisphenol S was characterized by electrospray mass spectrometry and nuclear magnetic resonance to confirm the structure.
[0065] Synthesis of unlabeled bisphenol S:
[0066] Weigh 100 mg of phenol into a 10 mL eggplant-shaped flask, drop 30 μL of 98wt% concentrated sulfuric acid into it, react at 125 °C for 2.5 h under nitrogen protection, and then heat up to 175 °C for 3 h; after the reaction, add 1 mL of methanol Dissolving the reaction product, the obtained mixture was concentrated under reduced pressure and separated and purified by normal phase preparative liquid chromatography. The elution reagents were petroleum ether and ethyl acetate, and the flow rate was 5 mL / min. Gradient elution was performed by volume ratio. The initial composition of the de-reagent is petroleum ether: ethyl acetate = 4: 1; when the elution is 25 min, the composition of the elutio...
Embodiment 2
[0075] synthesis 14 C-bisphenol S ([U-ring- 14 C12]-BPS):
[0076] Add 3.1 x 10 to the eggplant bottle7 Bq 14 C-phenol (11mg; 2.4×10 8 Bq / mmol, dissolved in 200 μL of petroleum ether), and then added dropwise 3.3 μL of 98wt% concentrated sulfuric acid, under nitrogen protection, reacted at 125 °C for 2.5 h, and then heated to 175 °C for 3 h; after the reaction, 1 mL of methanol was added to dissolve the reaction product, and the resulting mixture was concentrated under reduced pressure and separated and purified by normal-phase preparative thin-layer chromatography. The volume ratio to ethyl acetate is 1:1), the silica gel powder obtained by separation is extracted 5 times with ethyl acetate, the extract is concentrated under reduced pressure to remove ethyl acetate, and then analyzed by liquid chromatography-radioactive detector combined system ( The analytical conditions are shown in Appendix 2), the radioactive purity of the product is 98%, the yield is about 10.0%, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com