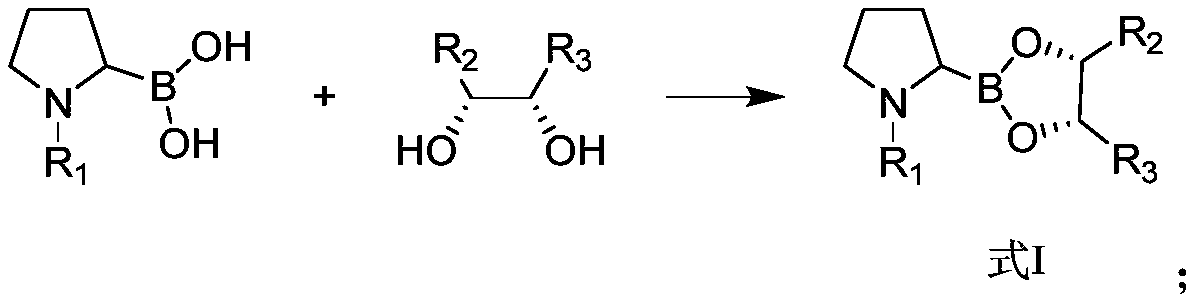
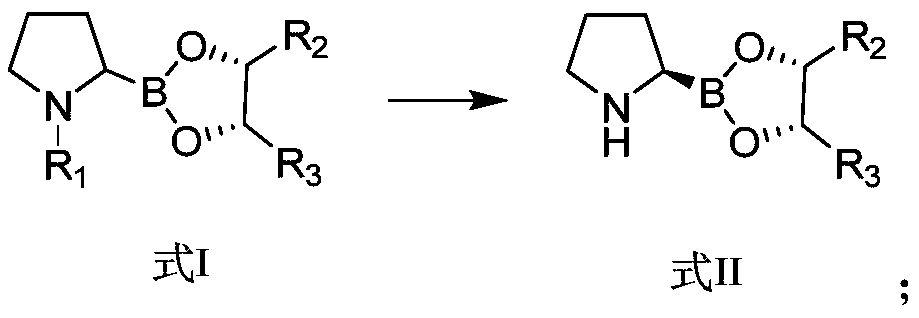
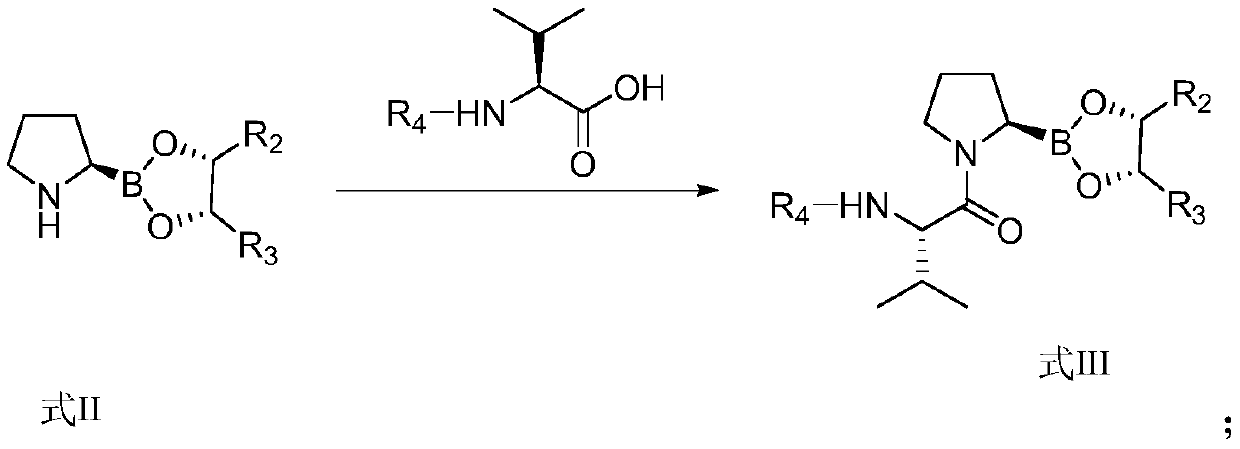
Preparation method of dipeptide valine boron proline salt
A technology of valine boroproline salt and valine, which is applied in the field of compound synthesis, can solve the problems of complex steps, low total yield of routes, and large losses, so as to simplify the post-reaction processing steps and increase the total yield , The effect of increasing the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] This example prepares a kind of [(2R)-1-[(2S)-2-amino-3-methylbutyryl]pyrrolidin-2-yl]boronic acid methanesulfonate
[0089] The synthetic route is
[0090]
[0091] (1) N-Boc-2-pyrrolidine boronic acid (215g, 1mol) was dissolved in tetrahydrofuran (1.5L), anhydrous magnesium sulfate (240g, 2mol) was added under stirring, and then (1S, 2S, 3R, 5S) - 2,3-pinanediol (170g, 1mol), after reacting for two hours, filtered, washed, and the organic phase was concentrated and dried, and intermediate A1 was obtained in 331g.
[0092] (2) Intermediate A1 (250 g) was dissolved in 1000 mL of ether, and hydrogen chloride gas was introduced at 0° C. for 30 min. Then the mixture was slowly warmed to room temperature and stirring was continued for 3 h. Cool to 0°C, and then direct rotary evaporation to obtain 204 g of white powder. The white powder was dissolved in water (1000 mL), and saturated aqueous sodium bicarbonate solution was added dropwise to pH=8.5. Then extract with d...
Embodiment 2
[0101]
[0102] Steps (1), (2), (3), (4) and (5) are the same as the preparation method of Example 1, except that the raw material acid HX in step (6) is hydrochloric acid.
[0103] (6) Dissolve 18g of intermediate E1 in 180mL of acetone, add hydrochloric acid (3.176g, 0.087mol) dropwise, stir at room temperature for 2h, filter, wash with acetone, ether, and dry to obtain (2R)-1- [(2S)-2-Amino-3-methylbutyryl]pyrrolidin-2-yl]borate hydrochloride (19.4 g, yield 92%).
[0104] 1 H NMR (MeOH-d 4 ,400MHz):δ4.03(d,1H),3.78-3.74(m,1H),3.49-3.42(m,1H),3.38-3.34(m,1H),3.34-3.19(m,1H),2.25 (ddd,1H), 2.16-2.05(m,2H), 2.04-1.92(m,1H), 1.80-1.67(m,1H), 1.13(s,3H), 1.08(s,3H).
Embodiment 3
[0106] This example prepares a kind of [(2R)-1-[(2S)-2-amino-3-methylbutyryl]pyrrolidin-2-yl]boronic acid methanesulfonate
[0107] The synthetic route is
[0108]
[0109] (1) N-Boc-2-pyrrolidine boronic acid (215g, 1mol) was dissolved in tetrahydrofuran (1.5L), anhydrous magnesium sulfate (240g, 2mol) was added under stirring, and then pinacol (118g, 1mol) was added, After reacting for two hours, it was filtered, washed, and the organic phase was concentrated and dried to obtain intermediate A2 in 276 g.
[0110] (2) Intermediate A2 (270 g) was dissolved in 1000 mL of ether, and hydrogen chloride gas was passed through at 0° C. for 30 min. Then the mixture was slowly warmed to room temperature and stirring was continued for 3 h. Cool to 0°C, and then direct rotary evaporation to obtain 201 g of white powder. The white powder was dissolved in water (1000 mL), and saturated aqueous sodium bicarbonate solution was added dropwise to pH=9. Then dichloromethane was extracte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com