Difenidol hydrochloride tablets and preparation method thereof
A technology of diphenidol hydrochloride and magnesium stearate, applied in the field of medicine, can solve problems such as low dissolution rate, and achieve the effects of small RSD of the drug, easy promotion and production, and improved dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Physicochemical properties of difenidol hydrochloride bulk drug: white crystalline powder; odorless; easily soluble in methanol, soluble in ethanol, slightly soluble in water or chloroform; pH value should be 5.0~7.0 ("China Pharmacopoeia 2015 Edition, Part II); melting point is 217-222°C ("Chinese Pharmacopoeia" 2015 Edition General Rule 0612, the first method), melting and decomposing simultaneously ("Chinese Pharmacopoeia" 2015 Edition Volume 2).
[0049] The Diphenidol hydrochloride bulk drug used in the following examples comes from Hunan Qianjin Xiangjiang Pharmaceutical Co., Ltd., which has impurity I(ene compounds, and its structure is shown in the following formula, 1-(4,4-diphenyl- 3-butenyl) piperidine, the chemical formula is C 21 h 25 N, the molecular weight is 291.44) shall not exceed 0.5%, and the sum of the peak areas of other impurities shall not be greater than 0.5% of the sum of the peak areas, which is equivalent to 34.59 mg of difenyl hydrochloride...
Embodiment 2
[0056] The preparation of embodiment 2 difenidol hydrochloride tablets
[0057] For every 1000 tablets of difenidol hydrochloride tablets, the specific components are: difenidol hydrochloride 25g, lactose 68g, starch 10g, pH regulator (potassium hydrogen tartrate) 16g, polyvinyl alcohol 1.6g, carboxymethyl cellulose Calcium 8g, magnesium stearate 1.3g.
[0058] The specific preparation process is as follows: pulverize difenidol hydrochloride, control the particle size of Dv90 between 10 and 50 μm, weigh the prescribed amount of difenidol hydrochloride, lactose, starch, potassium hydrogen tartrate, carboxymethyl cellulose Calcium is mixed evenly, and polyvinyl alcohol is weighed to form a slurry, and the evenly mixed material is added, after drying, the prescribed amount of magnesium stearate is added, mixed evenly, tableted according to the measured content, and coated.
Embodiment 3~8
[0059] The preparation of embodiment 3~8 difenidol hydrochloride sheet
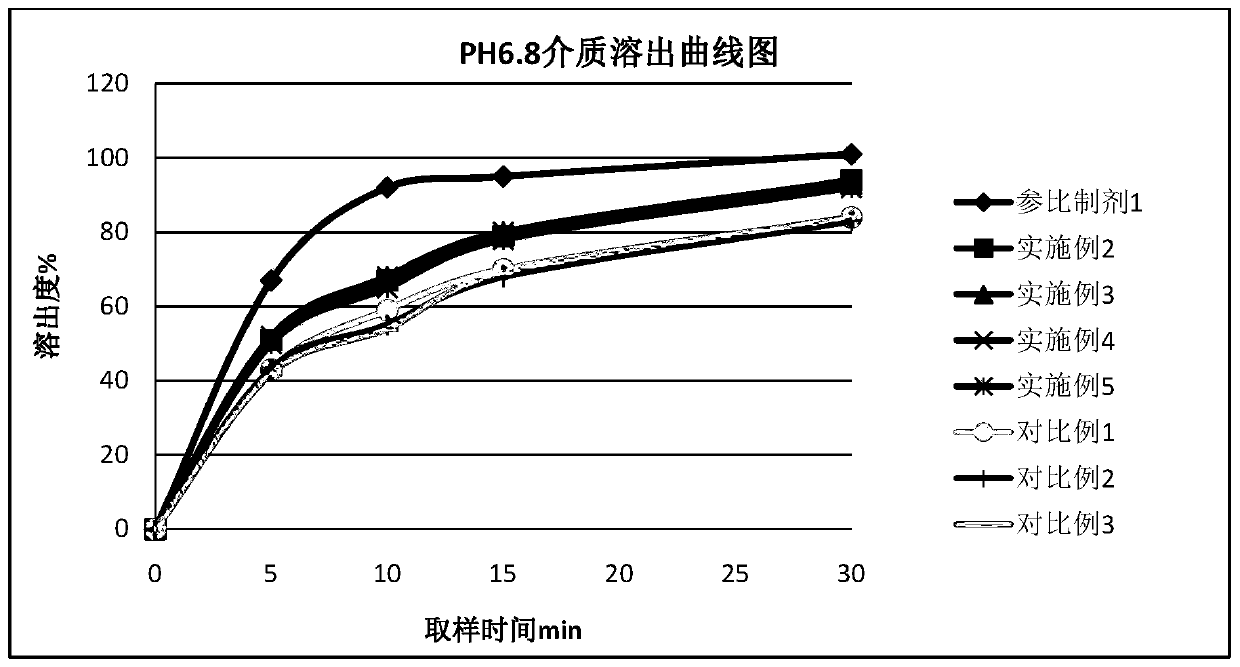
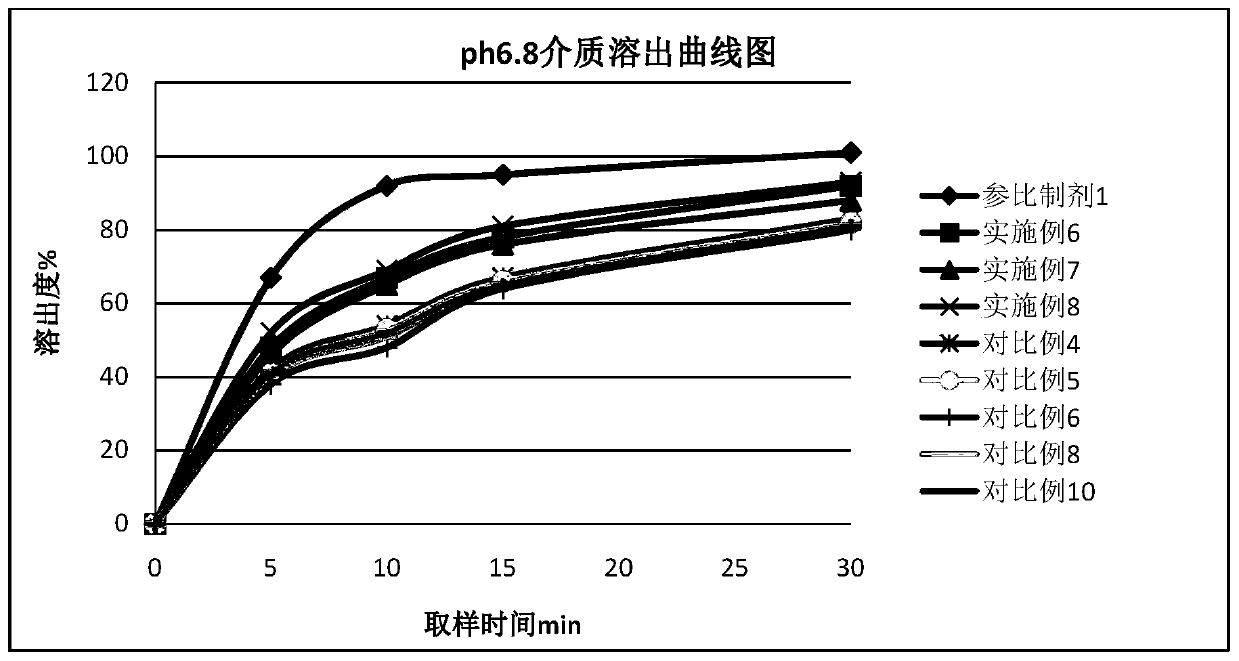
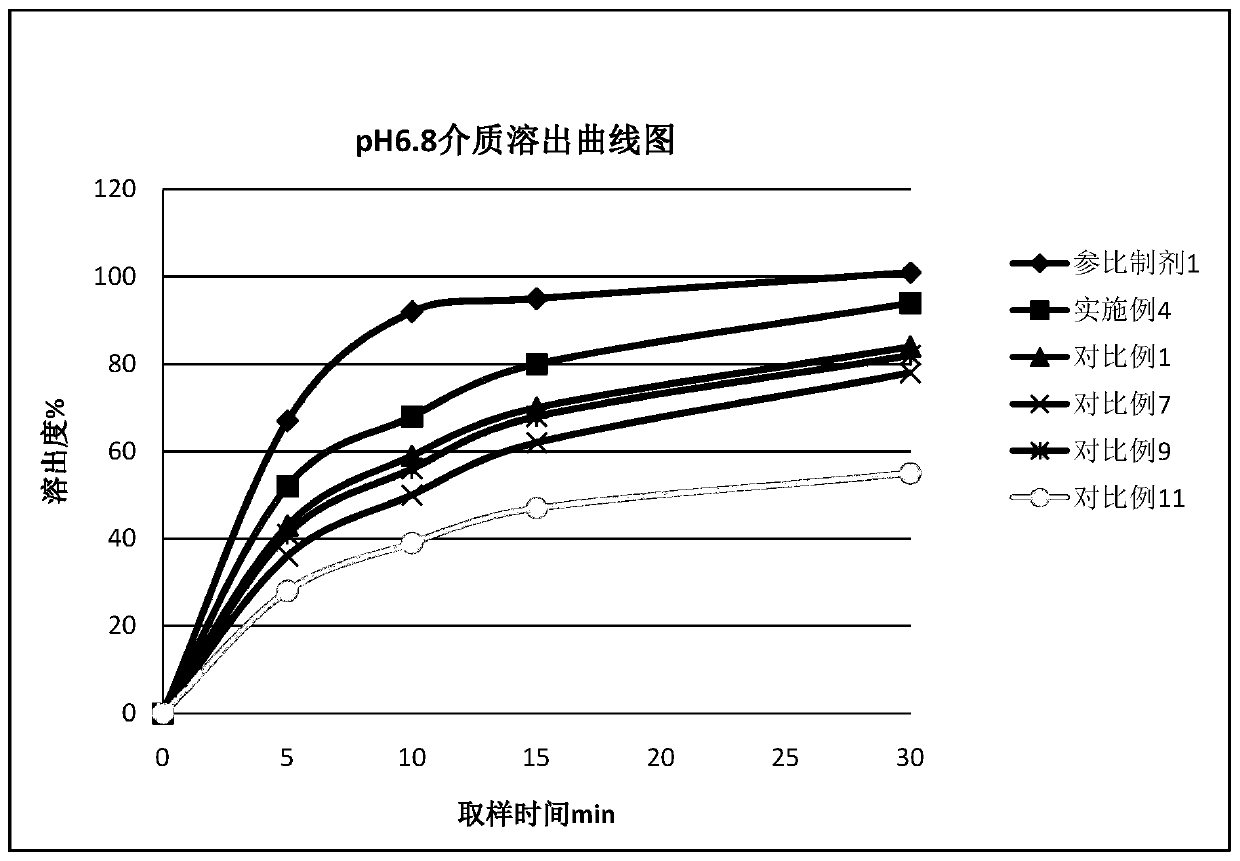
[0060] 1. According to the preparation method of Example 2, Diphenidol Hydrochloride Tablets were prepared according to the dosage of the components in Table 2, and at the same time, the formulation without adding pH regulators and the addition of different types and dosages of pH regulators were used as comparative examples. Difenidol Hydrochloride Tablets.
[0061] Table 2 Component types and dosage ratio
[0062]
[0063] 2, measure the pH value of the prepared difenidol hydrochloride tablet
[0064] The difenidol hydrochloride tablet prepared by the embodiment in table 2 and the difenidol hydrochloride tablet prepared by the comparative example are samples to be tested, and measure the pH value of its medicine. Samples with a ratio of 1 to 11 were prepared into a solution containing difenidol hydrochloride 2 mg / mL, and the pH value was measured according to the general rule 0631 of the fourth par...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com