Photosensitizer prodrug compound, and preparation method and application thereof
A technology of compounds and photosensitizers, applied in the field of medicine, can solve problems such as poor stability, restricting curative effect, dimerization and inactivation, etc., and achieve the effects of avoiding inactivation, promoting curative effect, and enhancing lipophilicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
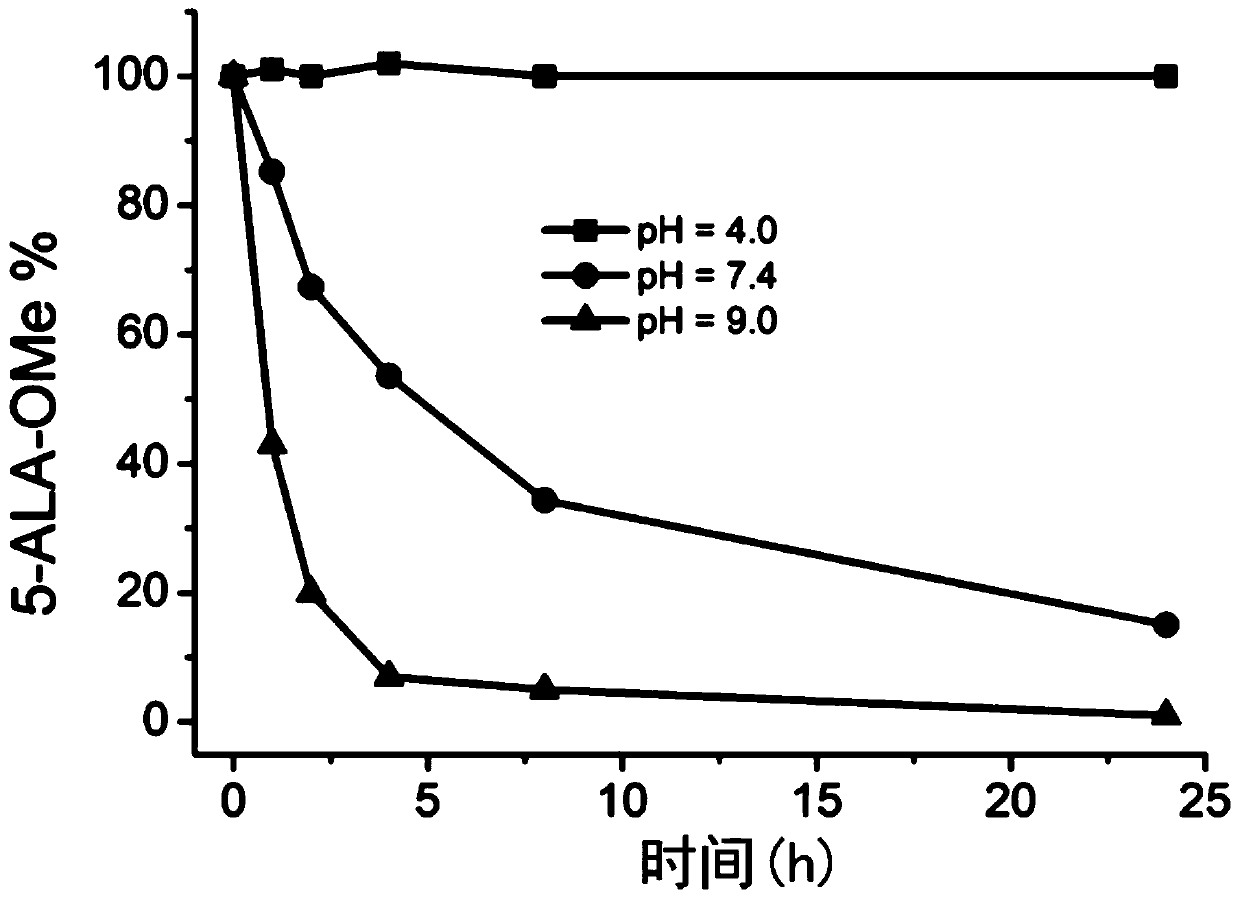
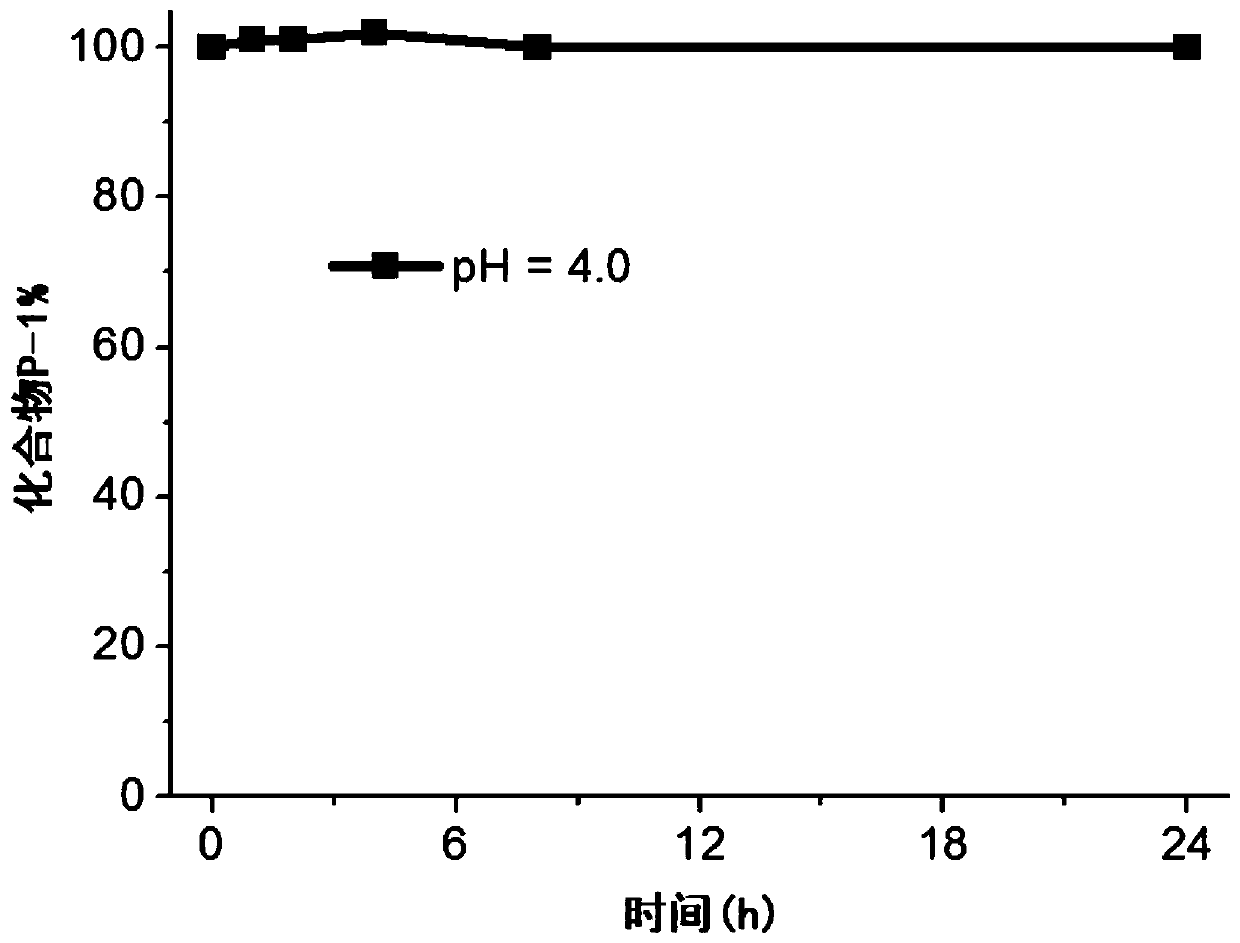
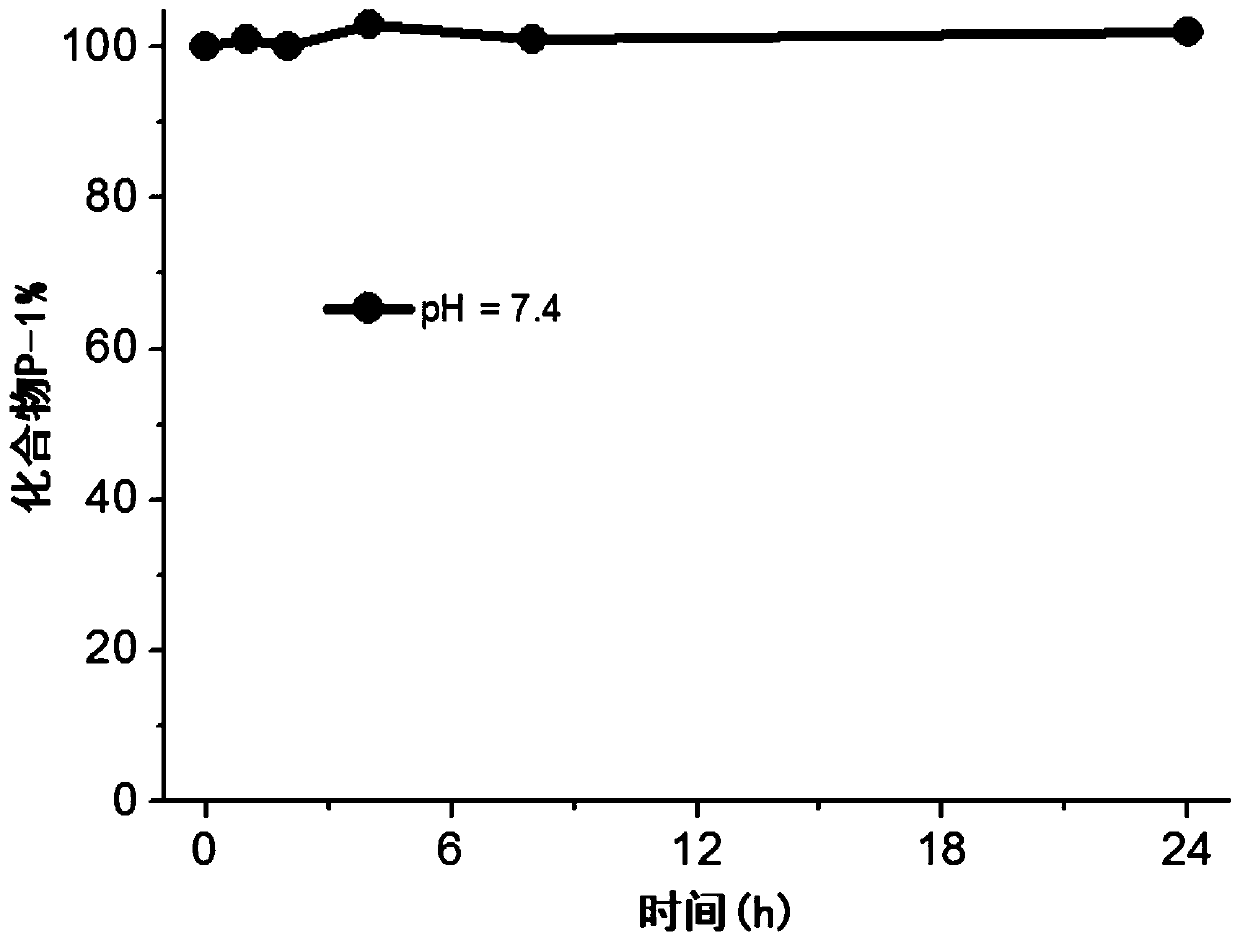
Image
Examples
Embodiment 1
[0058] This embodiment provides a photosensitizer prodrug compound (dimethyl 4,7,16,19-tetraoxo-8,15-dioxa-11,12-dithia-6,17-diazadocosanedioate), which has the following formula (P-1 ) shows the structure:
[0059]
[0060] The synthetic route of compound shown in formula (P-1) is as follows:
[0061]
[0062] The preparation method of the compound shown in formula (P-1) specifically comprises the following steps:
[0063] (1) Synthesis of Intermediate 1-1
[0064] 2-Hydroxyethyl disulfide (1.3mmol) was dissolved in ultra-dry acetonitrile (5mL), and under nitrogen protection, DSC (5.2mmol) and Et3N (7.8mmol) were added in sequence, and after 6 hours of reaction at room temperature, the solvent was spin-dried , the residue was dissolved in dichloromethane (20mL), and washed once with saturated sodium bicarbonate, saturated ammonium chloride and saturated saline solution, the organic phase was dried with anhydrous sodium sulfate, spin-dried to obtain intermediate 1-1; ...
Embodiment 2
[0071] This embodiment provides a photosensitizer prodrug compound, which has a structure shown in the following formula (P-2):
[0072]
[0073] The synthetic route of the compound shown in the formula (P-2) is the same as in Example 1, the difference is that the compound A-1 is replaced by 5-ALA, and the compound B-1 is replaced by a compound shown in the following formula (B-2) .
[0074]
[0075] The preparation method of the compound shown in formula (P-2) specifically comprises the following steps:
[0076] The steps of synthesizing intermediate 1-1 are the same as in Example 1;
[0077] The intermediate 1-1 (1.3mmol), the hydrochloride (1.3mmol) of 5-ALA and the hydrochloride (1.3mmol) of compound B-2 were dispersed in ultra-dry THF, and under nitrogen protection, DIPEA ( 3.9 mmol), after overnight reaction at room temperature, the solvent was spin-dried, the residue was dissolved in dichloromethane (20 mL), and washed once with saturated ammonium chloride, satu...
Embodiment 3
[0082] This embodiment provides a photosensitizer prodrug compound, which has a structure shown in the following formula (P-3):
[0083]
[0084] The synthetic route of the compound shown in formula (P-3) is the same as in Example 1, and the difference is that the compound A-1 is replaced by the compound shown in the following formula (A-3), and compound A-1 is replaced by the compound shown in the following formula (B-3). The compound shown is substituted for compound B-1.
[0085]
[0086] The preparation method of the compound shown in formula (P-3) specifically comprises the following steps:
[0087] The steps of synthesizing intermediate 1-1 are the same as in Example 1;
[0088] Disperse intermediate 1-1 (1.3mmol), compound A-3 hydrochloride (1.3mmol) and compound B-3 hydrochloride (1.3mmol) in ultra-dry THF, under nitrogen protection, slowly drop DIPEA (3.9mmol), after overnight reaction at room temperature, the solvent was spin-dried, the residue was dissolved ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com