Thioredoxin mutant, preparation method thereof and application of thioredoxin mutant in production of recombinant fusion proteins
A technology of thioredoxin and fusion protein, applied in the production of recombinant fusion protein, in the field of thioredoxin mutants, can solve unfavorable recombinant polypeptide or protein production, complicated purification process, large amount of organic solvent used, etc. problem, to achieve soluble stability, less denaturation, low-cost large-scale production, and good soluble stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Step 1) Establishment of thioredoxin mutant gene library
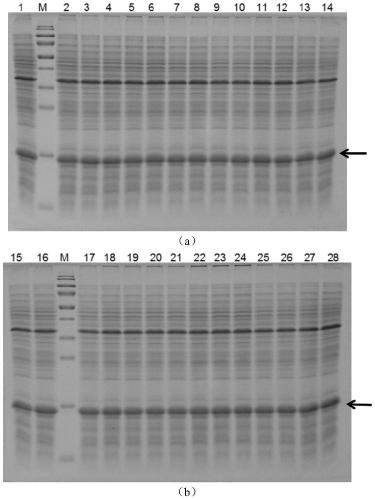
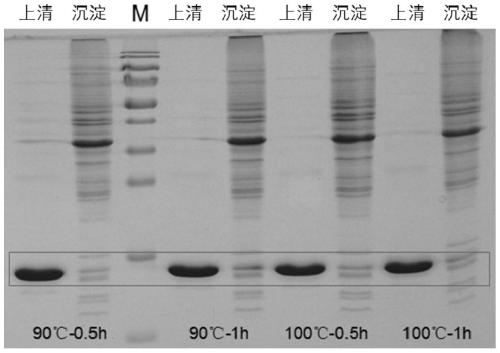
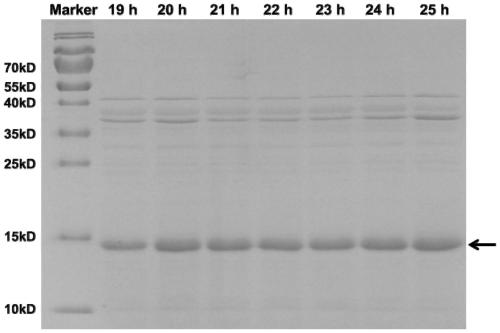
[0066] The amino acid sequence of thioredoxin (SEQ ID NO. 1) from Geobacillus stearothermophilu was optimized according to the codon preference of E.coli and synthesized by Universal Biosystems (Anhui) Co., Ltd., named TrxWT, and inserted into the vector pET28a. Multiple cloning sites were obtained to obtain the recombinant vector pET28a-TrxWT. Design primers and use the thioredoxin TrxWT gene as a template, perform error-prone PCR amplification, and insert the obtained random mutant fragment mixture into the vector pET28a to obtain a recombinant vector pET28a-TrxWT_epmut mix containing the mutant gene, and the recombinant vector mixture is transferred into Competent cells E.coli BL21 (DE3) were used to obtain an expression library with a library capacity of about 11000-12000. Fifty single colonies were randomly selected for double-enzyme digestion verification, and the results showed that about 25% were false ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com