Fluorescent probe and preparation method thereof
A fluorescent probe and naphthalene diimide technology, applied in the field of analysis and detection, can solve the problems of high cost, low use efficiency, and difficult long-term development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] An embodiment of the fluorescent probe of the present invention and its preparation method, the fluorescent probe of this embodiment
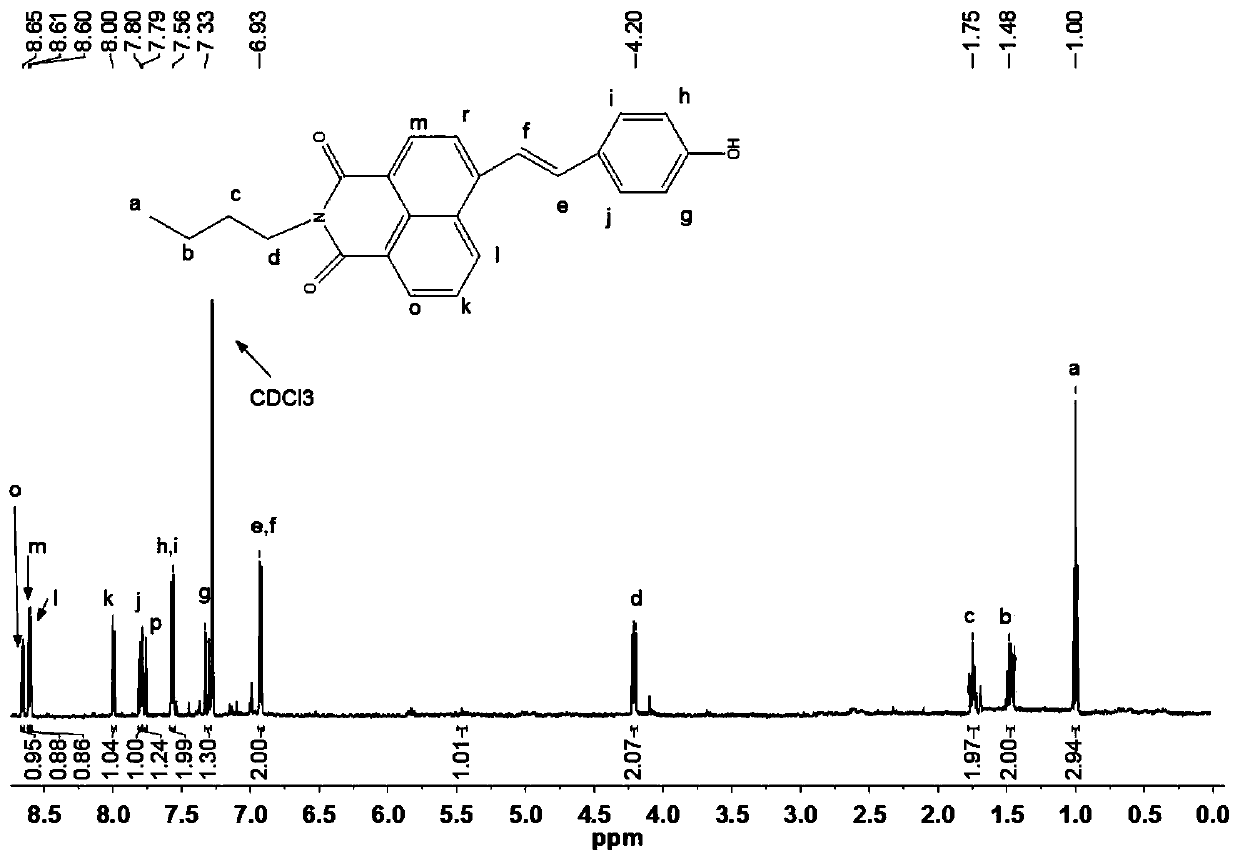
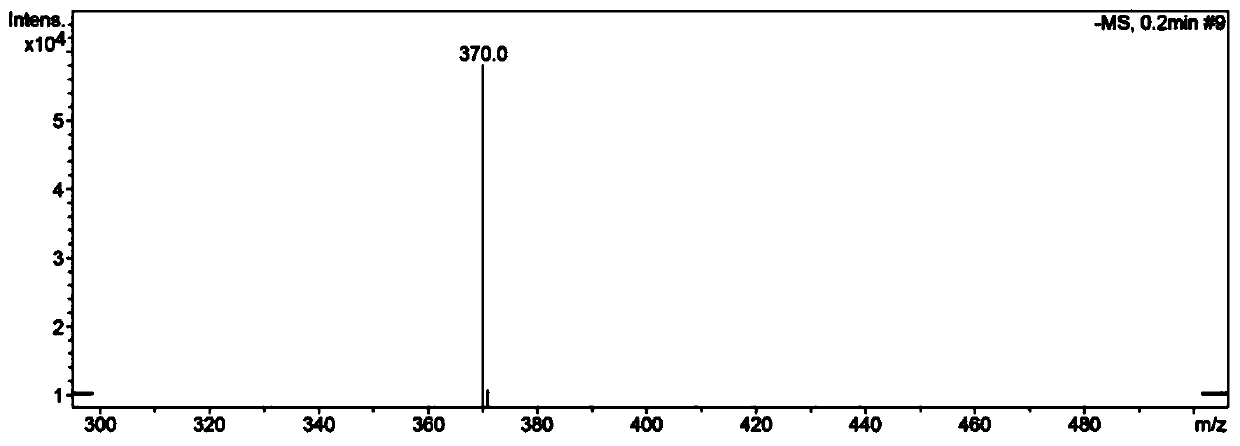
[0047] It is N-n-butyl-4-(4-phenol hydroxystyryl)-1,8-naphthalene diimide, and its structural formula is as Formula (I):
[0048]
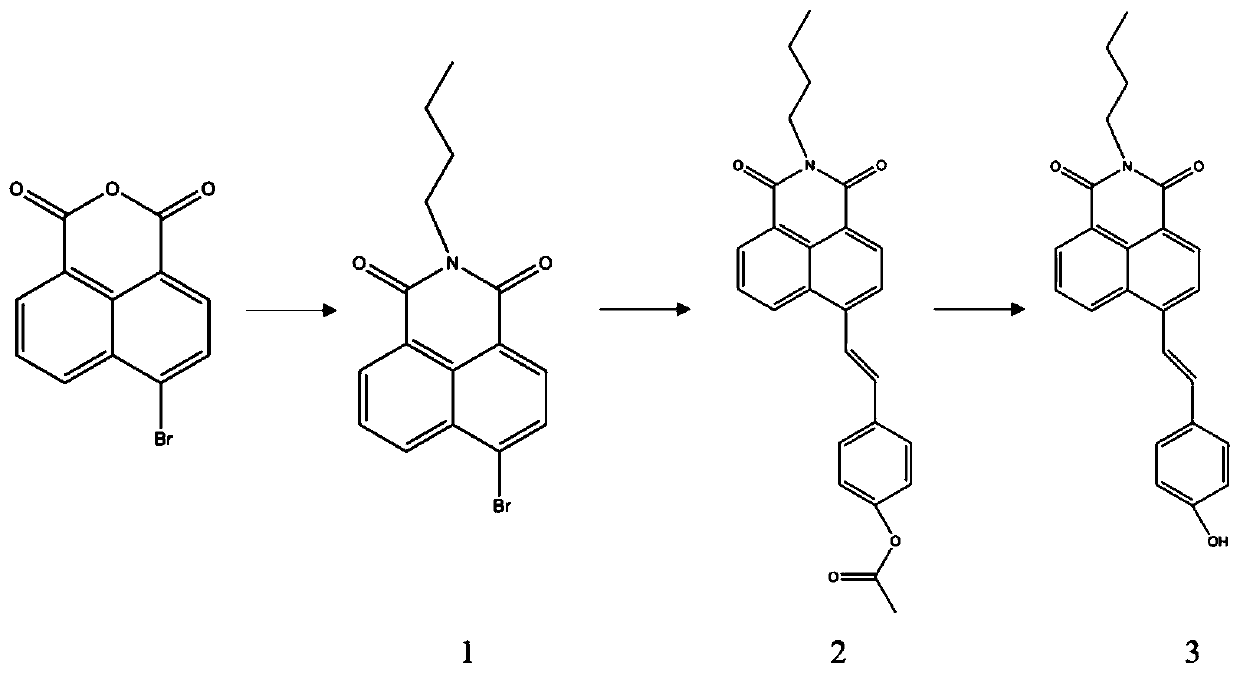
[0049] The fluorescent probe synthesis route of this embodiment is as follows figure 1 Shown, its specific preparation method is as follows:
[0050] 1) Under nitrogen protection and stirring conditions, stir and dissolve 4-bromo-1,8 naphthalene anhydride (500mg, 1.8mmol) and n-butylamine (450μL, 4.5mmol) in absolute ethanol (10-15ml) and use Heat the reaction solution to 85°C under oil bath conditions, connect the condenser to form reflux, and keep the reaction conditions for 3 to 4 hours, then stop heating. After the reaction solution is cooled to room temperature, take the reaction solution into a 250mL flask, and then use a rotary evaporator to The reaction solvent was evaporated, and the resulting ...
Embodiment 2
[0053] The action mechanism diagram of embodiment 2 fluorescent probe and isocyanate
[0054] This example tests the mechanism of action between N-n-butyl-4-(4-phenol hydroxystyryl)-1,8-naphthalene diimide fluorescent probe and isocyanate. The detection group of the fluorescent probe is a hydroxyl group, which is dissolved in an ethanol solution. After reacting with isocyanate under the condition of triethylamine, the hydroxyl group reacts to form a carbamate, and the ethanol solution of the fluorescent probe appears orange macroscopically. , the solution turns light green after interacting with isocyanate, the specific changes are as follows Figure 4 shown.
Embodiment 3
[0055] The ultraviolet fluorescence spectrum property of embodiment 3 fluorescent probes
[0056] In this example, the ultraviolet absorption and fluorescence spectra of N-n-butyl-4-(4-phenol hydroxystyryl)-1,8-naphthalene diimide fluorescent probe were tested. The specific method is as follows: Dissolve 4 mg of pure probe in 4 ml of absolute ethanol to prepare a standard probe solution (mother solution), take 300 μl into a cuvette, and then add 2.7 ml of absolute ethanol to prepare a detection solution. The detection solution is tested with a UV spectrophotometer to obtain a UV absorption spectrum as shown in Figure 5 As shown, the fluorescent probe has a strong absorption peak at 420nm; another 300 μl of the mother solution is taken into a cuvette, and then 2.7ml of absolute ethanol is added to prepare a detection solution, and the detection solution is subjected to fluorescence detection to obtain a fluorescent probe As shown in 9, the fluorescence spectrum of the fluores...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com