A polymer-based universal fluorescent platform for FRET fluorescent probe donors and its applications
A technology of fluorescent probes and polymers, which is applied in the fields of organic chemistry and polymer material chemistry, can solve problems such as complex synthesis processes, and achieve low cost, intuitive detection by naked eyes, and simple and easy synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025]
[0026] Synthesis of MS-4: In a solution of 6 g of rhodamine hydrazine hydrate (200 mg, 0.46 mmol) in dimethylformamide (about 3 mL), add phenyl isothiocyanate (94 μl, 0.69 μmol) with a molar mass of 1.5 times , gradually raised the temperature to 60°C, heated and stirred for four hours, and after TLC confirmed that the reaction was complete, the reaction solution was extracted with dichloromethane, the organic phase was rotary evaporated under reduced pressure, and the pink target product was obtained by solid column chromatography with a yield of 50%.
Embodiment 2
[0028]
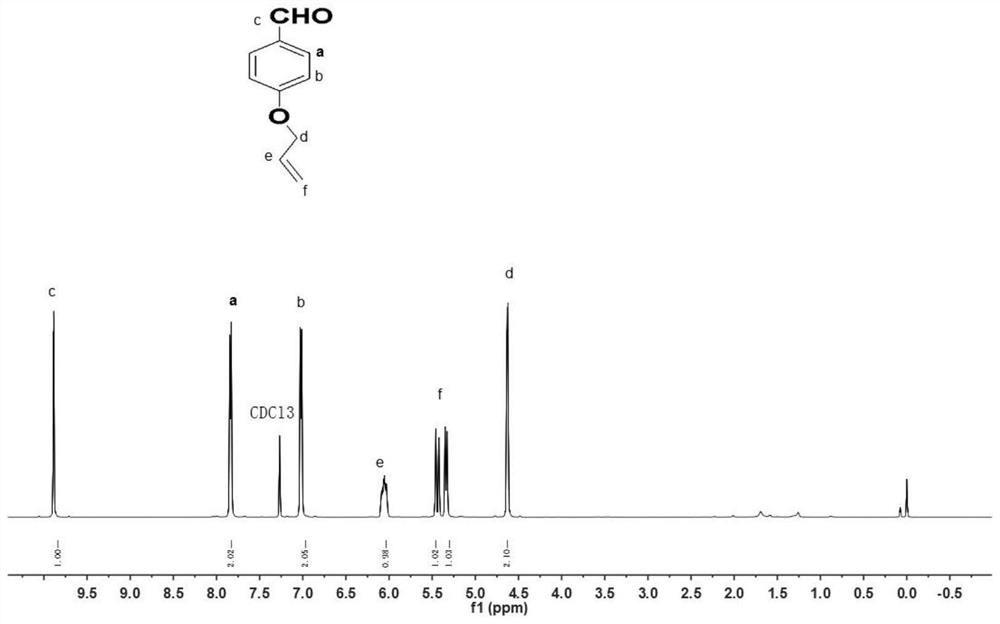
[0029] Synthesis of MS-3: 1 gram of p-hydroxybenzaldehyde, equimolar allyl chloride, and three times the molar amount of potassium carbonate were placed in a 50-ml flask, and 15 ml of acetonitrile was added. The reaction solution was heated to reflux for two hours, cooled and filtered, and the filtrate was spin-dried under reduced pressure to obtain a white solid powder (MS-3 p-allyloxybenzaldehyde), with a yield of 70%. The product was directly used in the next reaction without purification.
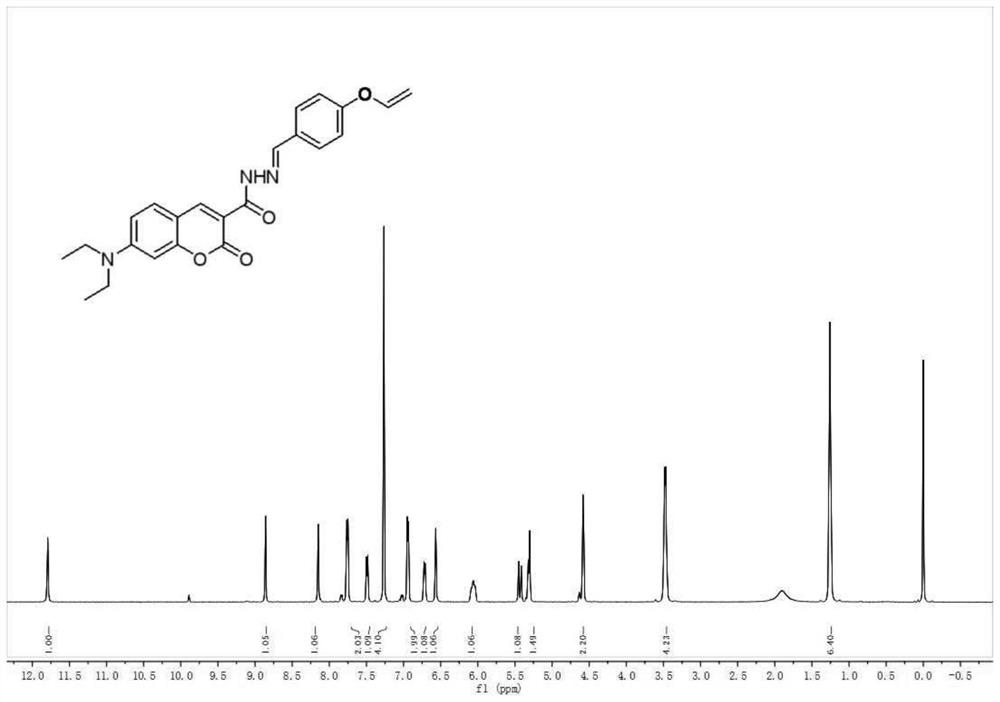
[0030] Synthesis of coumarin hydrazine hydrate: coumarin ester (1g, 3.46mmol) was dissolved in 15ml of methanol solution, slowly dripped into 2ml of hydrazine hydrate, the reaction solution was stirred at room temperature for 30 minutes, after TLC confirmed that the reaction was over, the precipitated solid was filtered 0.54 g of yellow solid powder was obtained, with a yield of 56%. The product was directly used in the next reaction without purification.
[0031] Synthesi...
Embodiment 3
[0033]
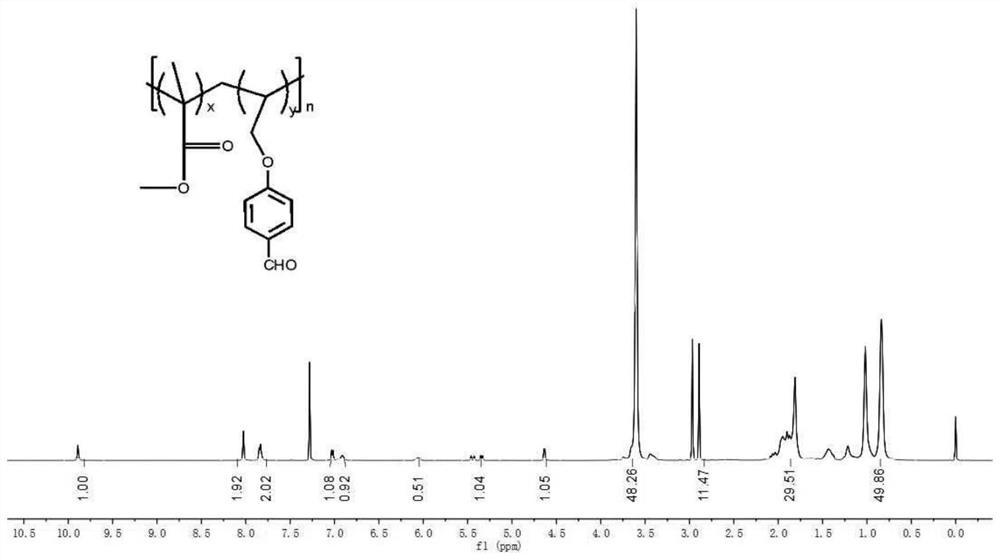
[0034] Synthesis of MS-2: Mix 5g methyl methacrylate (0.0499mol) and 3.3687g (0.0207mol) MS-3, and add 0.04g initiator azobisisobutyronitrile (0.0002mol) and 20mL dimethyl Formamide was bubbled with nitrogen for 10 min to remove oxygen from the solution. The reaction solution was placed in a constant temperature oil bath, and heated at 70° C. for 24 hours to obtain a transparent polymer. Then the reaction mixture was poured into methanol (200ml), and the polymer was precipitated in methanol, ultrasonically oscillated, filtered, washed with methanol several times to remove the unreacted monomer in the solid part, and the filtered polymer product was placed in a vacuum oven at 30°C Dry for 24 hours to constant weight. Yield: 4.1557 g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com