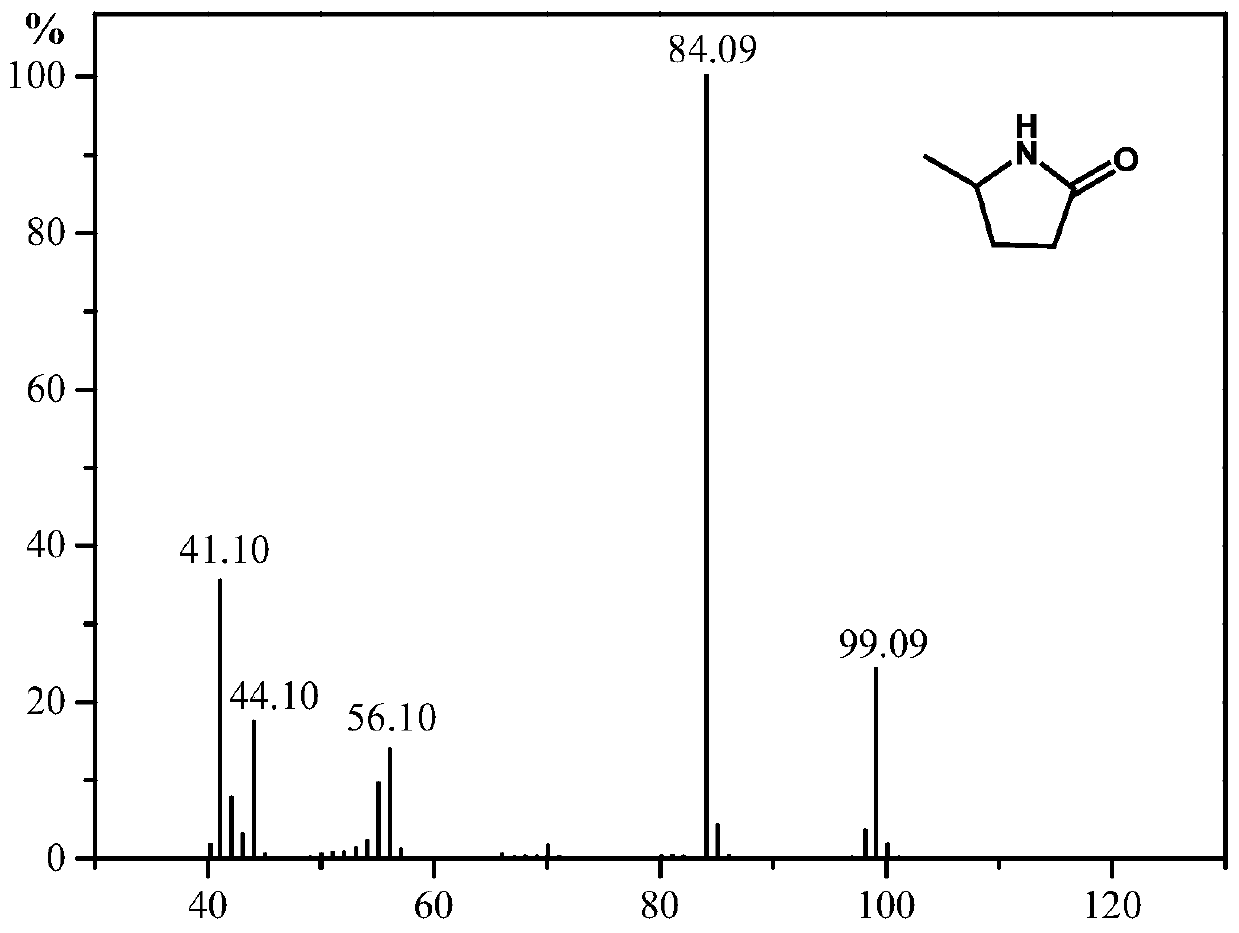
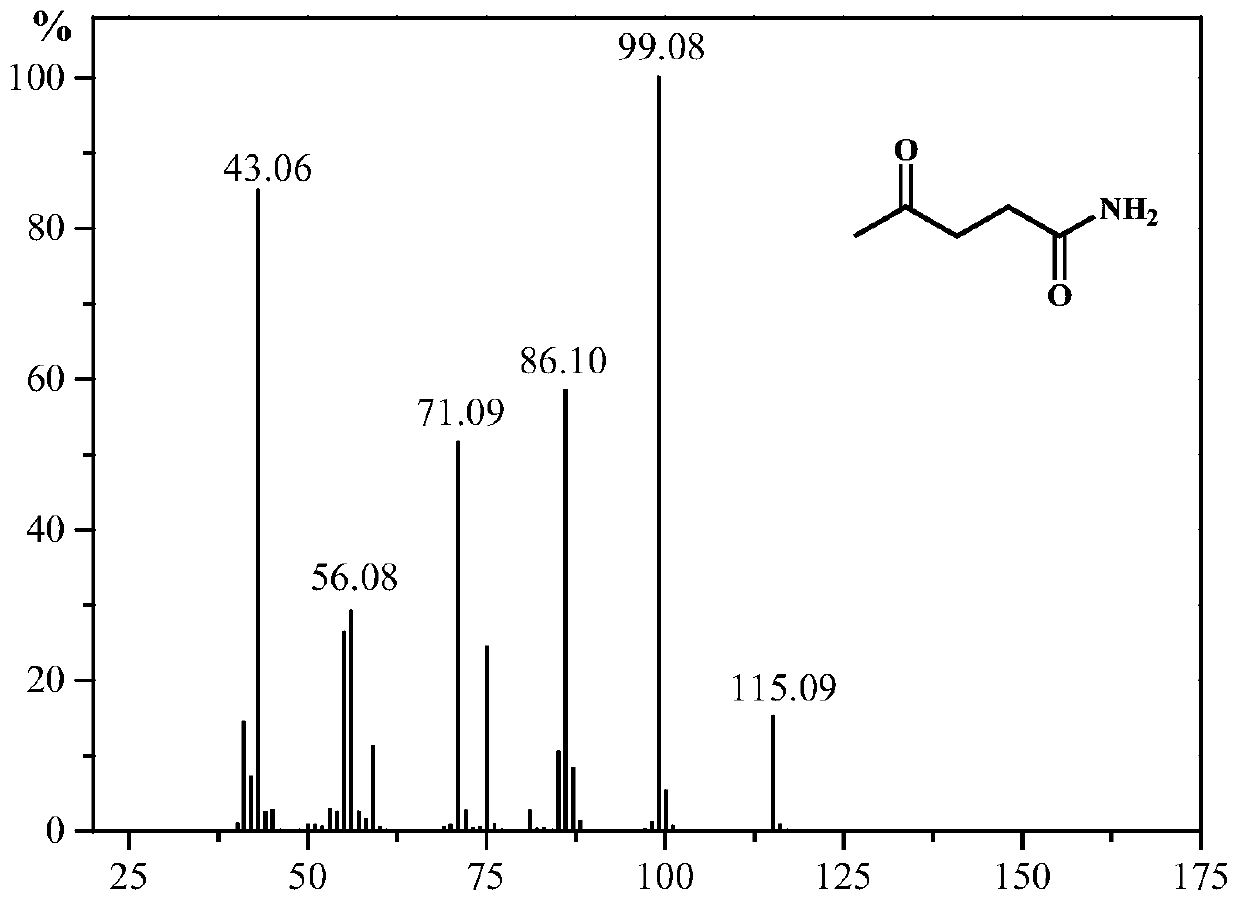
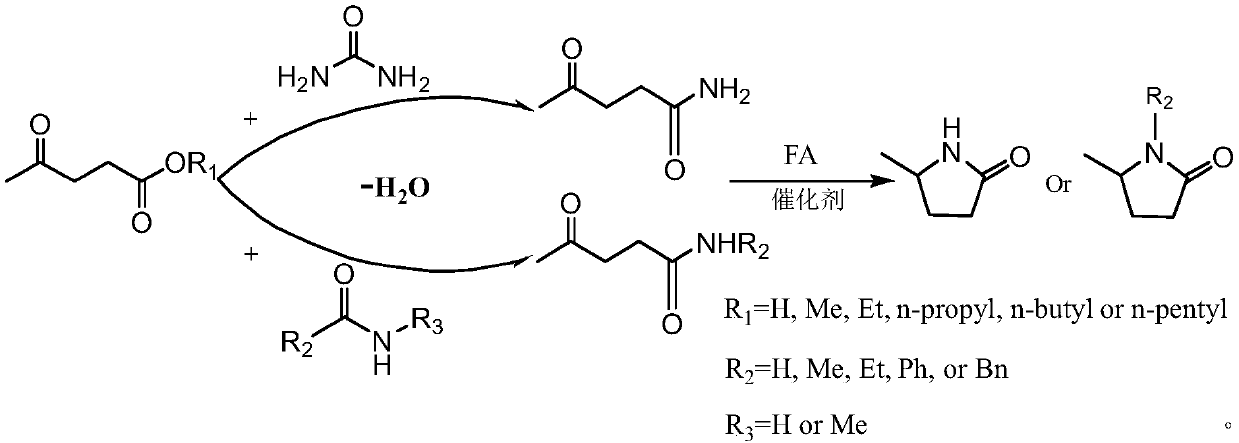
Synthesis method of 5-methyl-2-pyrrolidone or derivatives thereof
A pyrrolidone and synthetic method technology, applied in the field of organic catalytic synthesis, can solve the problems of product separation and purification difficulties, solvent volatilization and recovery, low product selectivity, etc., and achieve the effect of facilitating separation, avoiding side reactions, and simple reaction system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~6
[0031] Take 0.05mol of levulinic acid, methyl levulinate, ethyl levulinate, propyl levulinate, butyl levulinate, amyl levulinate respectively, and put them in high pressure reaction with equimolar urea and formic acid In the still, add 0.174g ruthenium carbon catalyst and mix evenly, seal the reaction kettle, heat to 130°C under magnetic stirring and keep for 6h respectively, finish the reaction and cool to room temperature and filter to recover the catalyst. GC-MS (Shimadzu) and GC (Agilent) were used for qualitative and quantitative detection, and the detection results are listed in Table 1 with serial numbers 1-6.
Embodiment 7~16
[0033] Take 0.1mol formamide, acetamide, propionamide, benzamide, phenylacetamide, N-methylformamide, N-methylacetamide, N-methylpropionamide, N-methylbenzamide respectively , N-methylphenylacetamide, put 0.05mol ethyl levulinate and 0.1mol formic acid in a high-pressure reactor, then add 0.29g platinum carbon catalyst and mix evenly, seal the reactor, heat to 100°C under magnetic stirring and Respectively keep 24h, finish the reaction and cool to room temperature and filter to recover the catalyst. GC-MS (Shimadzu) and GC (Agilent) were used for qualitative and quantitative detection, and the detection results are listed in Table 1 with serial numbers 7-16.
Embodiment 17~25
[0035] Take 0.05mol levulinic acid, 0.06mol formic acid, and 0.075mol urea in a high-pressure reactor, and then add 0.58g of rhodium carbon, palladium carbon, ruthenium carbon / copper oxide, platinum carbon / copper oxide, rhodium carbon / copper oxide , palladium carbon / copper oxide, platinum / molybdenum oxide, platinum / titanium dioxide, and platinum / zirconia catalysts were mixed evenly, and the reaction vessel was sealed, heated to 80°C under magnetic stirring and kept for 4 hours respectively. After the reaction was completed, the reaction was cooled to room temperature and the catalyst was recovered by filtration. GC-MS (Shimadzu) and GC (Agilent) were used for qualitative and quantitative detection, and the detection results are listed in Table 1 with serial numbers 17-25.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com