LAMP primers for detecting EB viruses in intraocular fluid and application thereof
An Epstein-Barr virus and primer set technology, applied in the biological field, can solve problems such as easy contamination, application limitations, and high false positive rate, and achieve high specificity, high sensitivity, and accurate detection effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Embodiment 1, the preparation of the primer set identifying Epstein-Barr virus
[0071] A large number of sequence analyzes and comparisons were carried out to obtain several primers for identifying Epstein-Barr virus. Preliminary experiments were performed on each primer to compare performances such as sensitivity and specificity, and finally a LAMP primer set for identifying Epstein-Barr virus was obtained.
[0072] A primer set for identifying Epstein-Barr virus, including 2 outer primers (F3-1, B3-1), 2 inner primers (FIP-1, BIP-1) and 2 loop primers (LF-1, LB-1 ), each primer sequence is as follows (5'→3'):
[0073] Primer F3-1 (sequence 1 of the sequence listing): GTTCGCGTTGCTAGGCC;
[0074] Primer B3-1 (sequence 2 of the sequence listing): AAGGGGAAAAGTTAGAAACTG;
[0075] Primer FIP-1 (Sequence 3 of the Sequence Listing): ATACCAGGGGCAGTGGTCCCTCTCAGTCCAGCGCGTTTA;
[0076] Primer BIP-1 (SEQ ID NO: 4 of the Sequence Listing): GTCCTGCAGCTATTTCTGGTCGCTAGGGAGAGGTAGA...
Embodiment 2
[0080] Embodiment 2, detection method establishment
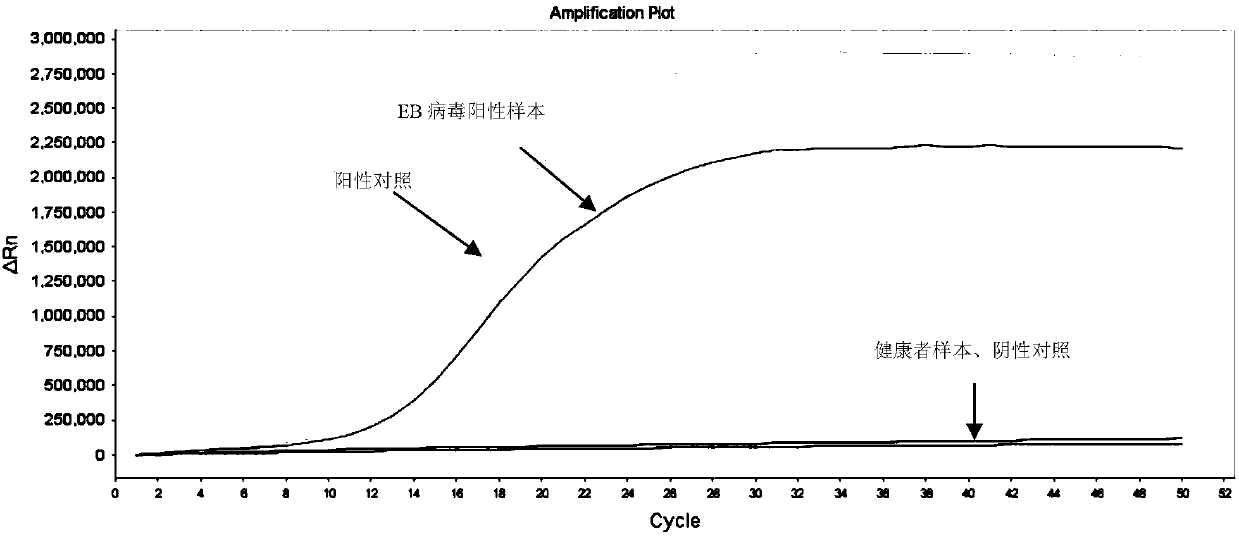
[0081] Utilize the method for the primer group detection Epstein-Barr virus of identifying Epstein-Barr virus of embodiment 1 to comprise:
[0082] 1. Extract the genomic DNA of the virus to be tested or extract the total DNA of the biological sample to be tested.
[0083] 2. Take the total DNA obtained in step 1 as a template, and use the primer set prepared in Example 1 to perform LAMP amplification.
[0084] The reaction system for 10μL LAMP amplification includes: 1μL 10×ThermoPol Buffer (Thermo), 0.8M Betaine, 0.5mg / ml BSA, 4mM MgSO 4 , 0.3μL 20×EvaGreen (Hefei Bomei Biotechnology Co., Ltd.), 1.5mM dNTPs, 0.32U / ml Bst DNA polymerase large fragment, primer mix (F3-1, B3-1, FIP-1, BIP-1, LF-1 and LB-1 mixture), 2ng template DNA, balance ddH 2 O. The concentration of each primer in the reaction system is as follows: 0.5 μM F3-1, 0.5 μM B3-1, 2 μM FIP-1, 2 μM BIP-1, 1 μM LF-1, 1 μM LB-1.
[0085] Reaction program for ...
Embodiment 3
[0088] Embodiment 3, identify the specificity of the primer group of Epstein-Barr virus
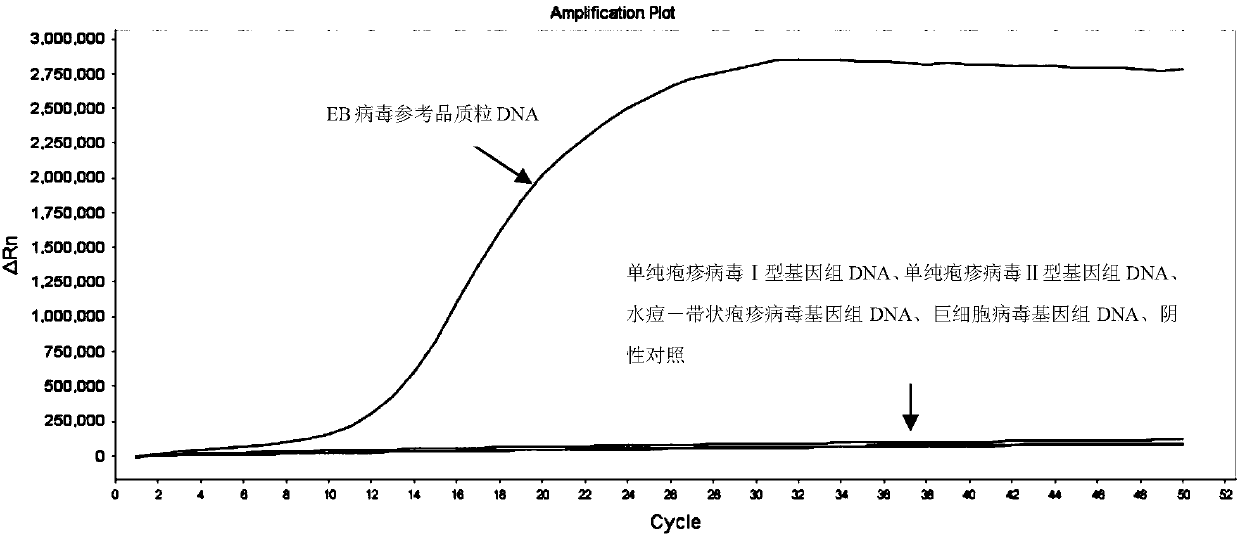
[0089] Epstein-Barr virus reference plasmid construction: insert the Epstein-Barr virus DNA fragment shown in Sequence 7 of the sequence listing between the ApaI and SacI restriction sites of the pGEM-TEasy Vector vector to obtain the reference plasmid.
[0090] The samples to be tested were: Epstein-Barr virus reference plasmid DNA, herpes simplex virus type I genomic DNA, herpes simplex virus type II genomic DNA, varicella-zoster virus genomic DNA, and cytomegalovirus genomic DNA. Among them, herpes simplex virus type Ⅰ, herpes simplex virus type Ⅱ, varicella-zoster virus and cytomegalovirus reference plasmid DNA are all recorded in the literature "Zhu Yanmin, Wu Yidong, Shang Shiqiang. Gene chip method for detection of seven herpes viruses and its preliminary application [J]. Journal of Clinical Laboratory, 2009 (5): 363-365.", the genomic DNA of each virus was extracted, and the sampl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com